CARDIOVASCULAR JOURNAL OF AFRICA • Advance Online Publication, November 2018
AFRICA
1
Pulmonary thromboendarterectomy in a combined
thrombophilia patient
Hakan Akbayrak, Hayrettin Tekumit
Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is a potentially correctable cause of secondary pulmonary hypertension. Surgical treatment remains the primary treat-ment for patients with CTEPH. Pulmonary thromboendar-terectomy (PEA) with deep hypothermic circulatory arrest is the standard and recommended surgical technique for the treatment of these patients. The prevalence of CTEPH after an acute pulmonary thromboembolism (PTE) has been found in various studies to be between 0.6 and 8.8%. Mortality rates in elective PEA cases with CTEPH are reported to be between 1.9 and 4.5%. We report on a 50-year-old female patient with combined inherited thrombophilia, including protein C and protein S deficiencies, who was diagnosed with CTEPH and was successfully treated with pulmonary thromboendarter-ectomy.
Keywords: protein C, protein S, pulmonary embolism,
thrombo-endarterectomy
Submitted 26/2/18, accepted 22/10/18
Cardiovasc J Afr 2018; 29: online publication www.cvja.co.za DOI: 10.5830/CVJA-2018-052
Chronic thromboembolic pulmonary hypertension (CTEPH) is the end result of untreated or recurrent pulmonary thromboembolism (PTE). The incidence of CTEPH is about 5% in patients who survive after acute PTE and up to 30% after recurrent PTE.1 After acute PTE, the incidence of cumulative
symptomatic CTEPH in the first six months was 1%, compared with 3.1% per year and 3.8% over two years.2 The prevalence
of CTEPH was shown to be between 0.6 and 8.8% in studies evaluating the prevalence of CTEPH after an acute PTE.3
CTEPH is currently largely under-diagnosed and must be sought in all patients presenting with exertional dyspnoea, reduction in effort capacity, fatigue, or clinical symptoms of right-sided heart failure. It can be seen with or without a prior history of deep venous thrombosis (DVT) and/or PTE. The patients with permanent symptoms of dyspnoea, fatigue, limitation of exercise, vertigo or chest pain following an acute PTE, as well as patients in whom pulmonary hypertension is reported as an acute event, could potentially benefit from further investigations to rule out CTEPH.1
Echocardiography, ventilation/perfusion (V/Q) scan, computerised tomography (CT), pulmonary angiography and cardiac catheterisation are standard diagnostic procedures.3 The
severity of the pulmonary hypertension determines the prognosis of CTEPH. If the mean pulmonary artery pressure (PAP) is greater than 30 mmHg, the five-year survival is approximately 30%. If the mean PAP is greater than 50 mmHg, the five-year life expectancy is estimated at 10%.1
V/Q scan is the preferred and recommended screening test for patients with CTEPH. Pulmonary angiography remains the gold standard for confirmation of chronic thromboembolic disease and evaluation of operability.2 It is recommended that all patients
with CTEPH should be evaluated with right heart catheterisation and pulmonary angiography to assess their prognosis.4
Surgical techniques have improved over the last 20 years but the standard surgical technique for PEA has not changed in the last five years. These procedures are currently safely performed in clinics with experienced surgeons.5,6
The progressive increase in pulmonary vascular resistance affects the clinical course of CTEPH. If left untreated, it could result in progressive pulmonary hypertension, right ventricular dysfunction and death.4
The standard treatment options for CTEPH patients are surgical treatment and/or balloon pulmonary angioplasty procedure (BPAP). BPAP can be performed on inoperable patients or as complementary treatment to surgery.5
The protein C system, which contains protein C, protein S and thrombomodulin, is a natural profibrinolytic system. Protein C and protein S are vitamin K-dependent plasma proteins that play a role in the negative feedback system in blood clotting. Thrombomodulin, which is a surface protein of endothelial cells, is also a part of the protein C system. Poor fibrinolytic activity is seen in protein C-deficient patients.7-9
Patients between 15 and 40 years who develop venous thrombotic complications, with a high incidence of DVT and PTE, usually have protein C and protein S deficiencies. Protein C- and protein S-deficient patients are treated medically with
Department of Cardiovascular Surgery, Faculty of Medicine, Selcuk University, Konya, Turkey
Hakan Akbayrak, MD, hakanakbayrak@gmail.com
Department of Cardiovascular Surgery, Faculty of Medicine, Bezmialem University, Istanbul, Turkey
Hayrettin Tekumit, MD
CARDIOVASCULAR JOURNAL OF AFRICA • Advance Online Publication, November 2018
2
AFRICA
heparin, warfarin, fresh frozen plasma, and protein C and protein S extract.7 We present a case of a surgically treated
CTEPH patient with protein C and protein S deficiencies.
Case report
A 50-year-old female had been admitted to another university hospital with exertional dyspnoea, palpitation and reduction in effort capacity. She had been treated with medical therapy only in that medical centre. Warfarin therapy had been started and continued after discharge.
When she applied to our hospital, her effort capacity was remarkably decreased, in New York Heart Association (NYHA) functional class IV. She also had leg swelling and sometimes haemoptysis. The patient was treated medically (bronchodilator therapy, glycocorticosteroid, furosemide, nitroglycerin, sildenafil) for severe dyspnoea and pulmonary hypertension in our cardiology and chest disease clinics.
Because of the development of her symptoms, she was referred to our clinic. Her weight was 65 kg and height was 165 cm. Blood pressure and pulse rate were in the normal range. The second heart sound was increased and widely split on chest auscultation. The lung sounds were normal. The liver was detected 5 cm below the right costal margin. There was mild bilateral leg oedema. The patient did not have any other risk factors such as smoking history, hormone use and family history. We did not find any thromboembolic focus for the pulmonary embolism in our patient.
A biochemical study revealed that the patient’s protein C activity was 34.9% (normal range: 70–150%) and protein S activity was 22.7% (normal range: 65–160%). Her partial oxygen pressure was 45 mmHg in room air on arterial blood gas analysis. The cardiothoracic ratio was 55% on chest X-ray. Her left ventricular ejection fraction was 60% and systolic PAP was 110 mmHg on echocardiography.
V/Q scan showed total occlusion of the right pulmonary artery (Fig. 1). Cardiac catheterisation showed that the systolic PAP was 110 mmHg and diastolic PAP was 9 mmHg (mean PAP 56 mmHg), right ventricular pressure was 110/0 mmHg (mean 25 mmHg), and pulmonary capillary wedge pressure was 10 mmHg. The pulmonary angiogram showed total right pulmonary artery occlusion. The patient was haemodynamically stable but urgent surgery was planned because of low partial oxygen pressure and
severe clinical symptoms.
After a median sternotomy, cardiopulmonary bypass (CPB) was established by cannulation of the aorta and two-stage caval cannulation. The patient was cooled to 18°C, the aorta was cross-clamped and cardiac arrest was established using antegrade blood cardioplegia and local cold application. The pulmonary arteries were explored by moving the aorta to the right side.
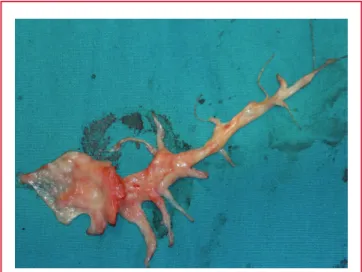
PEA was performed through right and left pulmonary incisions under total circulatory arrest (TCA) (Fig. 2). Firstly, we performed PEA on the right pulmonary artery and its branches within 20 minutes. There after we performed a left pulmonary artery incision for PEA in 12 minutes, but there were no thromboembolic material in the left pulmonary artery. The TCA time was 32 minutes and aortic cross-clamp time was 81 minutes. Ultrafiltration was performed on the patient and about 2 500 cm3
was filtered from the patient during CPB.
Alprostadil infusion and nitric oxide inhalation were administered after surgery. Alprostadil infusion was administered at a dose of 50 ng/kg/min for two days after the operation. Nitric oxide inhalation therapy was maintained until the patient was extubated at postoperative 18 hours. A low-molecular-weight
Fig. 2. The pulmonary thromboendarterectomy material that was removed from the right pulmonary artery.
Fig. 3. There were no perfusion defects in the lungs on V/Q scan after the operation.
Fig. 1. Pre-operative total perfusion defect in the right lung on V/Q scan.
CARDIOVASCULAR JOURNAL OF AFRICA • Advance Online Publication, November 2018
AFRICA
3
heparin was initiated at full dose within six hours of the end of the operation and continued up to the postoperative third day.
When the patient was extubated on the first day postoperatively, oral sildenafil and warfarin were started. A 3-l/min nasal oxygen tube was applied after the extubation. She was transferred to a general ward from the intensive care unit on the third day postoperatively.
Postoperative echocardiography, V/Q scan and computerised tomographic angiography (CTA) showed marked improvement in our patient. There were no perfusion defects in the lungs on the V/Q scan after the operation (Fig. 3). She was discharged uneventfully on the postoperative 10th day.
The patient was controlled at three, six, 12 and 24 months after the operation. She was evaluated according to NYHA functional class on echocardiography. She was in NYHA class I at the second-year check-up after the operation. The systolic PAP was 25 mmHg in the first year postoperatively. We designed the prothrombin time/international normalised ratio to range from two to three to control the warfarin effect postoperatively.
Discussion
PTE is normally eliminated by active fibrinolytic systems. Complete dissolution of a thromboembolism has been shown in four to eight days after a thromboembolic event in one study using pulmonary scanning. This study showed 0.5 to 4% of patients developed CTEPH, while 22% of patients continued to have signs of the disease.6 PEA is potentially the most successful
procedure for patients with CTEPH. These procedures are currently performed with low mortality rates in clinics with experienced surgeons.5,6
If chronic thromboembolic disease leads to CTEPH, it aggravates and leads to right ventricular failure due to a decline in vascular compliance across the pulmonary arterial circulation and increased vascular resistance.10 Thromboembolic disease
also leads to redistribution of blood flow within the pulmonary vasculature, resulting in the development of overflow and post-obstructive vasculopathy in the small pulmonary vessels, similar to that seen in pulmonary arterial hypertension.11 The
progressive increase in pulmonary vascular resistance affects the clinical course of CTEPH. If CTEPH is left untreated, it could result in progressive pulmonary hypertension, right ventricular dysfunction and death.4
Protein S acts as a co-factor to activated protein C to form the protein C–protein S complex. Thrombin generation via the inhibition of factor Va and factor VIIIa by binding to Ca2+ and
phospholipids is prevented by the protein C–protein S complex.9
Our patient had protein C and protein S deficiencies. A mortality rate of 5 to 9% seemed to be an acceptable risk for surgical treatment of her disease.7 Surgical treatment was planned
for our patient because of a poor prognosis on medical treatment. Control and prevention of recurrent PTE is very important. Pre-operative implantation of an inferior vena cava filter and
lifelong administration of warfarin is important to prevent recurrent attacks. In our patient, we did not use an inferior vena cava filter because she did not have acute or sub-acute DVT.
Conclusion
Patients diagnosed with CTEPH should have the diagnosis confirmed and the best therapeutic option determined according to the haemodynamic and morphological data provided by an invasive pulmonary angiogram and/or CTA. PEA for patients with CTEPH may be associated with acceptable peri-operative morbidity and mortality rates, and improved haemodynamic indices and survival rate.
This article was presented at the 16th National Congress of Vascular and Endovascular Surgery on 26–29 October 2013 in Istanbul, Turkey.
References
1. De Perrot M, McRae K, Shargall Y, Pletsch L, Tan K, Slinger P, et al. Pulmonary endarterectomy for chronic thromboembolic pulmo-nary hypertension: the Toronto experience. Can J Cardiol 2011; 27(6): 692–697.
2. Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350(22): 2257–2264. 3. Guérin L, Couturaud F, Parent F, Revel MP, Gillaizeau F, Planquette
B, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmo-nary embolism. Thromb Haemost 2014; 112(3): 598–605.
4. McNeil K, Dunning J. Chronic thromboembolic pulmonary hyperten-sion (CTEPH). Heart 2007; 93(9): 1152–1158.
5. Kim NH, Delcroix M, Jenkins DP, Channick R, Dartevelle P, Jansa P, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D92–99.
6. Lang IM, Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014; 130(6): 508–518.
7. Isoda S, Kimura T, Nishimura K, Yamanaka N, Nakamura S, Ando M, et al. A case report of pulmonary thromboendarterectomy for chronic thromboembolism in a patient with protein C deficiency. Ann Thorac Cardiovasc Surg 2014; 20(Suppl): 885–889.
8. Briffa NP, Wilson I, Clarke DB. Surgical treatment of pulmonary hyper-tension in protein C deficiency. Br Heart J 1991; 66: 460–462.
9. Mayo D, Zavada MC, Southerland CC Jr. The vascular adverse events of protein S deficiency: a case report. Ther Adv Cardiovasc Dis 2011; 5: 209–212.
10. Archibald CJ, Auger WR, Fedullo PF, Channick RN, Kerr KM, Jamieson SW, et al. Long-term outcome after pulmonary thromboen-darterectomy. Am J Respir Crit Care Med 1999; 160: 523–528. 11. Reichenberger F, Voswinckel R, Enke B, Rutsch M, El Fechtali E,
Schmehl T, et al. Long-term treatment with sildenafil in chronic throm-boembolic pulmonary hypertension. Eur Respir J 2007; 30: 922–927.