Environmental Pollution
H.N. ErtenAbstract The study of the environmental impact of natural and anthropogenic events forms the essence of environmental pollution considerations. The nature of the polluting species as well as their time distributions are of primary impor-tance with respect to identifying the polluting sources. Sediments are the most frequently used materials in such studies. Sediments act as the ecological memories of the environments of their formation. Besides classical chronological methods, radiochronological methods developed recently gave a big impetus to environmen-tal pollution studies. One of the key radioisotopic technique is to utilize210Pb, a product of the235U radioactive series to date the last 200 years of the sediments. A number of supporting indicators are also utilized. One of them being anthro-pogenic137Cs which is used as a time-marker. Large amounts of137Cs radioactivity was released to the environment during 1954–1963, in time of the most intense atmospheric nuclear arms testing and again in 1986 during the Chernobyl nuclear accident. These intense 137Cs activities form time-markers throughout sediment cores corresponding to their release years. During our studies extending over sev-eral years we have used radiochronological methods in dating sevsev-eral sediment cores from Zurich and Constance Lakes in Switzerland from east coast of Spain, Sea of Marmara, from the Black Sea region, Southern coast of Turkey and from North Cyprus. The distribution of several elemental concentrations of importance in pollution considerations along sediment cores were also determined. These stud-ies allowes proposing time frames to pollution events and help inquerstud-ies in tracing possible sources of pollution.
Keywords Environment· Pollution · Radiochronology
H.N. Erten (B)
Department of Chemistry, Bilkent University, 06800 Ankara, Turkey e-mail: erten@fen.bilkent.edu.tr
1015 H. Gökçeku¸s et al. (eds.), Survival and Sustainability, Environmental Earth Sciences,
1 Introduction
The formation of our world and its age has been a curiosity of mankind since ancient times. With the discovery of radioactivity in 1896 the age of the world has been accurately determined using radioisotopic techniques as 4.5× 109years. The nat-ural radioactive series of238U,235U and232Th and their decay products as well as cosmic ray produced radionuclides have been used for accurate dating of archeo-logical artifacts. These techniques were also useful in establishing a time frame for the impact of natural and anthropogenic activities on the environment. During over-ground nuclear weapons testing significant amounts of radioisotopes were emitted to the atmoshere. These kinds of activities were most intense in the years 1953–1954 and 1963–1964. Also big nuclear accidents such as Chernobyl in 1986 led to the sharp increases in radioactivities of the various environmental samples. Because of its high fission yield and long half-life the radioisotope137Cs has been used as a convinient time marker.
Radiochronological dating of sediments from various acquatic environments combined with the measurement of of their chemical profiles allow the study of the impact of natural and cultural events on the surrounding environment. The radionu-clide210Pb (t1/2= 22.3 y), a member of the natural radioactive decay series of238U, provide a reliable possibility of dating sediments over the last 200 years [1]. This technique has since seen further refined and very widely used by many researchers [2–11]. This method of dating, requires the complete recovery of the topmost sed-iment layers.7Be (t1/2= 53.3 d) a cosmic ray produced nuclide is expected to be present only in the uppermost sediment layers due to its short half-life. The presence of7Be in the sediments thus ensures complete core recovery.
We have used the above mentioned radioisotopic methods over the years for the dating of sediments from Lakes Zurich and Constance, the sea of Marmara, southern Turkey, eastern Spain, the Black Sea region and northern Cyprus [5–11].
Elemental distributions along the sediment samples were determined using spec-trocopic techniques such as XRF, AA. Utilizing the measurements together with the dating results allowed the determination of the time of pollution and their possible sources.
2 Dating Methods
2.1
210Pb Method
The commonly used radioisotope for dating sediments, 210Pb, is a member of the natural radioactive series 238U (t1/2= 4.4 × 109 y). 210Pb is incorporated into the sediments through the following processes. The noble gas nuclide222Rn (t1/2 = 3.82 d), a member of the 238U decay chain, diffuses out of the earth’s crust and is emmitted to the atmosphere. Radon gas decays completely while in the atmoshere leading to decay products such as210Pb.210Pb radioisotopes returns
to land and water surfaces with a constant flux as a result of wet and dry precip-itation. In water marine environment210Pb is scavenged by suspended particulate matter and accumulates in the sediments. Following the activity of210Pb across sed-iment cores leads to the determination of sedsed-imentation rates and hance dating of the sediment cores over a time scale of 200 years.
2.2
137Cs Method
As a result of extensive nuclear weapons test (1953–1964) and nuclear accidents (Chernobyl 1986) the distribution pattern of fission product137Cs exhits peaks cor-responding to these dates across sediment cores. The positions of these time marker peaks in the sediments all calculations of sedimentation rates. This complementary dating method may be used together with210Pb dating.
2.3
7Be Method
Cosmic ray produced radionuclibe7Be (t1/2= 53.3 d) is incorporated into the sed-iments via the same mechanisms as210Pb and137Cs. Because of its short half-life however; the radioactivity of7Be can only be detected in the uppermost layers of sediments. Measurement of7Be in sediments thus ensures that no loss occurs in the upper parts of sediment cores during the sampling process. Any such loss leads to serious errors in sedimentation rates and thus corresponding dates.
3 Experimental
Sediment cores were recovered using either a gravity corer or a box corer. The cores were immediately sampled in 0.5, 1 and 2 cm intervals after recovery. Part of the core sections were used for textural, mineralogical and chemical studies.210Pb was determined through its daughter210Po (t1/2= 138.4 d) in radioactive equilibrium with its parent. Polonium was distilled from the sediment at 600◦C and was con-verted to the chloride form by several evaporations with HCl. It was taken in a 0.5 M HCl solution. SO2 gas was bubbled through the solution for 3 minutes at 93◦C. Polonium was self-deposited on a silver disc which was suspended in the hot solution.
One side of the disc was coated with RUTEX, liquid rubber, ensuring deposition on one side only. The overall chemical yield was 90% as determined by the208Pb tracer. Alpha activities were determined using surface-barrierα-detectors. The210Po activities were converted into210Pb activities using standard growth and decay equa-tions. In some cases210Pb was determined directly byγ-rayspectrometry via the 46.5 keV line using a hyperpure Ge detector with 92 cm2active area. The137Cs and7Be activities were determined byγ-ray spectroscopy using a well-type Ge (Li) detector.
4 Results and Discussion
Sedimantation rates obtained by the above mentioned methods from various regions are summarised in Table1. The sedimentation rates obtained by three different methods in the dating of Lake Zurich sediments agree quite well with each other. Annual varves were not observed in the sediment samples from other regions. Sediment traps were used in Lake Constance and the rates obtained agree with those from210Pb and137Cs methods. The sedimentation rates at different regions of the Mediterranean and Black Sea given in Table1are considerably higher than those from the lakes.
This probably arises due to the fact that the sediment cores were recovered very near the shores. Table2 gives the distribution of trace elements across sediment cores of Lake Zurich sediments [12].
The trace element levels is seen to be low and somewhat constant before industrialization and gradually reaching maximum values with the beginning of industrialization. It is further observed that due to strict controls the trace element levels start to decrease after 1980. We analyzed the distribution of var-ious trace elements throughout the sediment cores of the sea of Marmara using ICP-AES.
Figure1shows the distribution of some of these trace element along the sediment depth.
The elements Zn, Cu, P and possibly Pb show near surface enrichment corre-sponding to the last 200 years as found by210Pb dating. Since no significant changes were observed in organic and inorganic contents in the sediment cores;enrichment by natural causes can be ruled out. This suggests anthropogenic causes for the observed increases. Towards the end of the eighteenth century, great changes were introduced in the Ottoman Army and Navy. New foundries, armament works, and shipyards were being constructed in and around ˙Istanbul. These activities may be responsible for the enrichment of the above mentioned metallic elements in the sediment cores.
Table 1 Summary of sedimentation rates obtained in our studies at various regions, using different dating methods
Mass sedimentation rate(g.cm2.y−1)
Sediment core 210Pb method 137Cs time marker Varve counting Sediment traps Lake Zurich 0.073± 0.015 0.07± 0.01 0.07± 0.02 – Lake constance 0.11± 0.02 0.09± 0.01 – 0.14± 0.09 Sea of Marmara 0.087± 0.012 – – – Southern Turkey 0.083± 0.013 – – – East Spain 0.21± 0.02 0.13± 0.02 North Cyprus 0.17± 0.03 0.19± 0.02 Black sea 0.22± 0.02 0.17± 0.3
Table 2 Distributions of trace elements across sediment cores from Lake Zurich [12] Sediment depth (cm) Calender year Cu (ug/g) Zn (ug/g) Pb (ug/g) Cd (ug/g) Hg(ug/g)
0.3 1989 48 224 69 1.1 0.6 1988 45 235 68 1.0 0.9 1987 42 182 57 0.75 1.5 1985 47 218 72 1.0 1.8 1984 38 197 62 0.8 2.4 1982 44 258 86 1.0 3.6 1978 49 259 108 1.5 3.9 1977 37 232 97 1.7 0.3 4.5 1975 46 273 102 2.5 5.1 1973 37 300 105 2.5 0.4 5.4 1972 46 308 109 2.4 5.7 1971 55 375 130 3.2 6.2 1969 55 475 137 4.0 0.7 6.5 1968 51 398 116 2.6 6.8 1967 52 300 107 6.5 7.4 1965 61 675 144 6.25 0.7 8.2 1962 78 587 104 13.4 8.6 1960 67 375 125 nd 9.5 1957 67 476 116 19.1 10.1 1955 65 350 150 6.2 1 10.7 1953 56 429 106 10.9 11.5 1950 65 170 77 2.4 0.6 11.8 1949 56 347 134 5.4 13.1 1945 55 250 120 4.5 0.7 15.0 1936 53 270 111 4.7 15.3 1935 55 375 142 4.5 17.5 1930 202 130 2.0 19.5 1925 47 167 105 1.2 0.5 20.3 1921 47 205 138 1.3 20.6 1920 57 202 115 1.7 22.3 1918 62 165 122 1.0 0.6 24.5 1910 67 150 112 1.0 27.5 1902 65 150 112 1.0 0.5 28.0 1900 77 117 112 1.0 29.7 1896 57 82 115 0.5 0.7 32.5 1886 32 60 17 0.2 40.5 1839 27 55 40 0.2 0.4 47.0 1819 20 55 12 0.2 52.5 1801 22 50 10 0.2
Another interesting example of the environmental impact on sediments was found in the Black Seas Samples. The distribution of some trace elements deter-mined by XRF along the Black Sea sediments is shown in Fig.2.
It is observed that at about 20 cm depth there is a noticable increase in these ele-mental concentrations. This depth corresponds to about 120 years as determined by radiochronology. Examining different anthropogenic factors causing the increase it was noticed that the Creamian war (1854–1856) with intense sea and land fights
Depth (cm) Fig. 1 Distrubition of some
trace elements along sediment depth of sea of marmara sediments
took place during this time. Thus suggesting a possible cause of the observed increases.
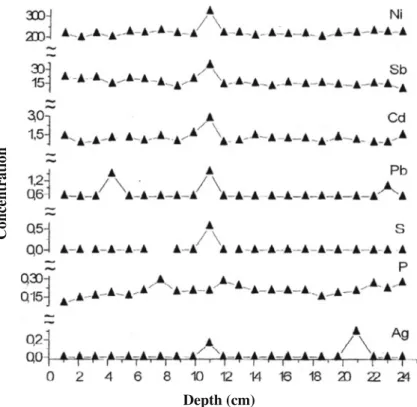
The trace element distribution in the sediment cores from southern Turkey deter-mined by XRF spectroscopy is shown in Fig. 3. The increase in the elemental concentrations at 11 cm depth may be attributed to the industrialization activities in this region. The trace element distrubitions across the sediment depth in the sediment samples from North Cyprus are shown in Fig.4.
Concentration
Depth (cm)
Fig. 2 The distribution of Ni, Co, Cr, Ca, Mg and S determined by XRF, along the Black sea sediments
Concentr
a
tion
Depth (cm)
Concentration
Depth (cm)
Concentration
Depth (cm) Fig. 4 Trace element distributions in the sediment cores from North Cyprus
A steady increase in elemental concentrations is observed at 24 cm depth cor-responding to about 80 years and continue until the surface. Cyprus in known to be have rich reserves of copper mines. After 1974 the mining companies stoped their activities. As a result the region become a serious environmental disaster area. The mining activities as well as the remains of the copper mine works may be the primary sources of elemental pollution observed.
References
1. Goldberg ED (1963) Radioactive Dating. IAEA STI/PUB/68, Vienna, Austria, p 121 2. Krishnaswami S, Lal D, Martin JM, Meybeck M (1971) Geochronology of lake sediments.
Earth Planet Sci Let 11:407–414
3. Robbins JA, Edgington D (1975) Determination of recent sedimetation rates in lake Michigan using Pb-10 and Cs-137. Geochim Cosmochim Acta 39:285–304
4. Smith PP, Valton A (1980) Sediment accumulation rates and geochronologies measured in the Saguenay Fjord using the Pb-210 method. Geochim Acta 44:225–240
5. Erten HN, von Gunten HR, Rössler E, Sturm M, Schweiz Z (1985) Dating sediments from lake Zurich (Switzerland) with210Pb and137Cs. Schweiz Z. Hydrol 47:5–11
6. von Gunten HR, Sturm M, Erten HN, Rössler E, Wegmüller F (1987) Sedimentation rates in the central lake Constance determined with210Pb and137Cs. Schweiz Z Hydrol 49:275–283 7. Evans G, Erten HN, Alavi SA, von Gunten HR, Ergin M (1989) Superficial deep-water
sediments of the Eastern Marmara basin. Geo-Marine Lett 9:27–36
8. Tadjiki S, Erten HN (1994) Radiochronology of sediments from Mediterranean sea using natural210Pb and137Cs. J Radionanal Nucl Chem 181:447–459
9. Gökmen A, Yıldız M, Erten HH, Saliho˘glu I (1996) Dating of sea of Marmara sediments by a uniform mixing model. J Environ Radioactivity 33:91–104
10. Erten HN (1997) Radiochronologies of lake sediments. Pure Appl Chem 69(1):71–76 11. Ayçık GA, Çetaku D, Erten HN, Salihlio˘glu I (2004) Dating of Black sea sediments from
Romanian coast using natural Pb-210 and fallout Cs-137. J Radioanal Nucl Chem 259:177– 180
12. von Gunten HR, Sturm M, Moser RN (1997) 200-year record of metals in lake sediments, their history, influence factors, regional differences. Environ Int. 31:63–75

![Table 2 Distributions of trace elements across sediment cores from Lake Zurich [12]](https://thumb-eu.123doks.com/thumbv2/9libnet/5982780.125441/5.659.86.571.104.737/table-distributions-trace-elements-sediment-cores-lake-zurich.webp)