In vitro cytotoxicity of compounds isolated from Desbordesia glaucescens
against human carcinoma cell lines
V. Kuete
a,b,⁎
, F.L. Dongmo Mafodong
c, I. Celik
d, S.A.T. Fobofou
c,e, B.L. Ndontsa
c, O. Karaosmano
ğlu
b,f,
L.A. Weissjohann
e, P. Tane
c, A.T. Koparal
b, H. Sivas
ba
Department of Biochemistry, Faculty of Science, University of Dschang, Dschang, Cameroon bDepartment of Biology, Science Faculty, Anadolu University, Eskişehir, Turkey
c
Department of Organic Chemistry, Faculty of Science, University of Dschang, Dschang, Cameroon dDepartment of Chemistry, Faculty of Science, Anadolu University, Eskişehir, Turkey
eDepartment of Biorganic Chemistry, Liebniz Institute of Plant Biochemistry, Weinberg 3, 06120 Halle (Saale), Germany f
Department of Biology, KamilÖzdağ Science Faculty, Karamanoğlu Mehmetbey University, Karaman, Turkey
a b s t r a c t
a r t i c l e i n f o
Article history:
Received 14 October 2016
Received in revised form 21 December 2016 Accepted 9 March 2017
Available online 20 March 2017 Edited by L Verschaeve
Malignancies constitute a global health concern and chemotherapy remains the main mode of treatment. The present study was designed to evaluate the cytotoxicity of 8 compounds from Desbordesia glaucescens namely lanosta-7,24-dien-3-one (1), friedelanone (2), friedelanol (3), 3,3′-di-O-methylellagic acid (4), 3,3′,4′-tri-O-methylellagic acid (5), ellagic acid (6), 3′,4′-di-O-3,3′,4′-tri-O-methylellagic acid 4-O-β-D-glucopyranoside (7) and
3,3′-di-O-methylellagic acid 4′-O-β-D-xylopyranoside (8) against 4 human carcinoma cell lines and normal CRL2120 fi-broblasts. The neutral red uptake (NRU) assay was used for cytotoxicity testing. Caspase-Glo assay, cell cycle anal-ysis, measurements of mitochondrial membrane potential (MMP) and levels of reactive oxygen species (ROS) were used to evaluate apoptosis induction. Compounds 4 and 6 as well as doxorubicin had IC50values below
45μM in the four tested cancer cell lines meanwhile other compounds displayed selective activity. The IC50values
ranged from 11.23μM (towards breast adenocarcinoma MCF-7 cells) to 44.65 μM (colon carcinoma Caco-2 cells) for 4, from 14.07μM (towards MCF-7 cells) to 77.73 μM (Caco-2 cells) for 6 and from 0.07 μM (towards SPC212 cells) to 1.01μM (A549 cells) for doxorubicin. Compound 4 induced apoptosis in MCF-7 cells mediated by MMP loss. The constituents of Desbordesia glaucescens and especially ellagic acid (6) and its derivative 4 are potential cytotoxic compounds that deserve more investigations towards developing novel antiproliferative drugs against human carcinoma.
© 2017 SAAB. Published by Elsevier B.V. All rights reserved.
Keywords: Apoptosis Carcinoma Cytotoxicity Ellagic acid Terpenoids Mode of action 1. Introduction
Cancer is one of thefive leading causes of death globally and accord-ing to the International Agency for Research on Cancer (IARC) for 2012, 14.1 million new cancer cases and 8.2 million deaths occurred in 2012 worldwide (Bray et al., 2013; Ferlay et al., 2013). The most occurring cancers in both developed and less developed countries include lung, breast, colon prostate, liver, stomach and cervix cancers (Torre et al., 2015). Chemotherapy remains the major mode of the treatment of var-ious neoplastic diseases. Although there have been vast discoveries of potent cytotoxic agents from plants, there is a growing need of novel cy-totoxic drugs to diversify the armory of anticancer agents and to offer
new therapeutic alternatives (Zeytinoglu et al., 2003; Ipek et al., 2005;
Zeytinoglu et al., 2008; Unlu et al., 2010). Drug discovery from African
plants is of relevant interest because Africa hosts 57,704 species of the world'sflora (Fadeyi et al., 2013). In the past, evidence of the anticancer activity of several plants and compounds from Africanflora was docu-mented (Kuete and Efferth, 2015). In our continuous search of cytotoxic molecules to combat malignant diseases, the present study was de-signed to investigate the antiproliferative potential of compounds iso-lated from Desbordesiaglaucescens (Engl.) Tiegh. (Irvingiaceae). Desbordesiaglaucescens is found around forest zones in South Nigeria, West Cameroon, Gabon, Congo, and Angola (Letouzey, 1982). In Congo, the seeds are used as a food additive while bark-decoctions are taken to attenuate stomach-ache, and as an aphrodisiac. Ointments made with barks and palm-oil are used to treat chicken-pox and head-ache (DongmoMafodong et al., 2015). Previous phytochemical studies on D. glaucescens reported the isolation of ellagic acid derivatives and triterpenes (DongmoMafodong et al., 2015).
⁎ Corresponding author at: Department of Biochemistry, Faculty of Science, University of Dschang, Dschang, Cameroon.
E-mail address:Kuetevictor@yahoo.fr(V. Kuete).
http://dx.doi.org/10.1016/j.sajb.2017.03.031
0254-6299/© 2017 SAAB. Published by Elsevier B.V. All rights reserved.
Contents lists available atScienceDirect
South African Journal of Botany
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / s a j b2. Materials and methods 2.1. General procedure
Mass spectral data [Electrospray ionization mass spectrometry (ESI-MS)] were measured on a Waters Synapt HDMS spectrometer. NMR Spectra were recorded with an Agillent DD2 NMR (400 MHz) spectrom-eter. Column chromatography was performed on silica gel Merck 60 F254[(0.2–0.5 mm) and (0.2–0.063 mm)] 70–230 and 230–400
mesh (Darmstadt, Germany). Pre-coated silica gel 60 F254thin layer
chromatography (TLC) plates (Merck, Germany) were used for moni-toring fractions and spots were detected with UV light (254 and 365 nm) and then sprayed with 50% sulphuric acid (H2SO4) followed
by heating to 100 °C. 2.2. Plant material
The stem of Desbordesia glaucescens (Irvingiaceae) were collected in Suza (Littoral Region-Cameroon) in March 2012. The plant material was authenticated (voucher number 53939/HNC) at the National Herbarium in Yaoundé by a botanist (Mr. Mezili Paul).
2.3. Extraction and isolation
Air-dried and powdered leaves (2.6 kg) of D. glaucescens were ex-tracted with EtOH (10 L) at room temperature for 48 h andfiltered. Thefiltrate was concentrated to dryness under vacuum to give 158.7 g of crude extract (CE). The obtained extract was suspended in distilled water and subjected to a liquid–liquid extraction using EtOAc and n-butanol to give ethyl acetate (EAF), and n-n-butanol (n-ButF) fractions, respectively.
Part of crude extract (151 g) was suspended in distilled water and subjected to a liquid–liquid extraction using EtOAc to give 50 g of ethyl acetate (EAF) and 82 g n-butanol fractions (n-ButF), respectively. Ethyl acetate (EAF) fraction (47 g) was subjected to CC over silica gel and eluted with a gradient of n-Hex-EtOAc and EtOAc-MeOH of increas-ing polarity. 59 fractions of 300 mL each were collected as follows: n-Hex (frs1–6); n-Hex-EtOAc (90:10) (frs7–23); n-Hex-EtOAc (85:15) (frs24–28); n-Hex-EtOAc (80:20) (frs29–38); n-Hex-EtOAc (60:40) (frs39–41); n-Hex-EtOAc (30:70) (frs42–44); EtOAc (frs45–51); EtOAc-MeOH (50:50) (frs52–57); MeOH (frs58–59). They were then combined into four sub-fractions based on their thin layer chromatogra-phy (TLC) profile: EAFA (09 g; frs1–8); EAFB (08 g; frs9–32); EAFC (11 g; frs33–44) and EAFD (12 g; frs45–59).
EAFA (07.5 g) was subjected to CC over silica gel and eluted with n-Hex-CH2Cl2mixture of increasing polarity. 53 new sub-frs of 75 mL each
were collected. n-Hex (sub-frs1–12); n-Hex-CH2Cl2(95:05)
(sub-frs13–19); n-Hex-CH2Cl2 (90:10) (sub-frs20–25); n-Hex-CH2Cl2
(85:15) (sub-frs26–33); n-Hex-CH2Cl2 (80:20) (sub-frs34–37);
n-Hex-CH2Cl2 (60:40) (sub-frs38–41); n-Hex-CH2Cl2 (50:50)
(sub-frs42–45); CH2Cl2−MeOH (50:50) (sub-frs46–51); MeOH
(sub-frs52–53). They were then combined into five sub-fractions based on their thin layer chromatography (TLC) profile: EAFA1 (0.7 g; sub-frs1– 14); EAFA2 (1.3 g; sub-frs15–21); EAFA3 (2.3 g; sub-frs22–31); EAFA4 (1.7 g; sub-frs32–44) and EAFA5 (0.7 g; sub-frs45–51). sub-fr EAFA1 (1–14) and sub-fr EAFA2 (15–21) was further subjected to silica gel CC eluting with n-Hex-CH2Cl2mixture of increasing polarity to afford
1 (230 mg). The ordinaryfiltration of sub-fr EAFA3 (22–31) afforded 2 (60 mg). The sub-fr EAFA4 (32–44) was further subjected to another sil-ica gel CC eluting with n-Hex-CH2Cl2(90:10) isocratic system to afford
(3; 16 mg).
EAFC (10 g) was subjected to CC over silica gel and eluted with n-Hex-AcOEt mixture of increasing polarity. 69 new sub-frs of 100 mL each were collected. The ordinaryfiltration of sub-frs 49–55 afforded white powder identified 3,3′-di-O-methylellagic acid (4; 26 mg).
n-Butanol fraction (n-ButF) (77 g) was subjected to CC over silica gel and eluting with mixture solvent of EtOAc-MeOH of increasing polarity. 44 fractions of 300 mL each were collected as follows: EtOAc (frs1–11); EtOAc-MeOH (90:10) (frs12–20); EtOAc-MeOH (80:20) (frs21–32); EtOAc-MeOH (70:30) (frs33–36); EtOAc-MeOH (50:50) (frs37–39); MeOH (frs40–44). They were then combined into five sub-fractions based on their thin layer chromatography (TLC) profile: n-But A (11 g; frs1–11); ButFB (18 g; frs12–27); ButFC (20 g; frs28–36) and n-ButFD (20 g; frs37–44).
Fraction n-ButFA (11 g) was subjected to CC over silica gel and elut-ed with CH2Cl2-MeOH mixture of increasing polarity. 72 new sub-frs of
75 mL each were collected as follows: CH2Cl2(sub-frs1–7); CH2Cl2
-MeOH (95:05) (sub-frs8–20); CH2Cl2-MeOH (90:10) (sub-frs21–35);
CH2Cl2-MeOH (80:20) (sub-frs36–55); CH2Cl2-MeOH (50:50)
(sub-frs56–70); MeOH (sub-frs71–72). They were further pooled into 4 other sub-frs according to their TLC profiles: n-ButFA1 (0.8 g; sub-frs1–14); n-ButFA2 (1.8 g; sub-frs15–31); n-ButFA3 (2.4 g; sub-frs32– 53); and n-ButFA4 (2.1 g; sub-frs54–72). sub-fr n-ButFA3 (23–38) was further subjected to CC over silica gel and eluted with an isocratic sol-vent system [CH2Cl2-acetone (90:10)] to give 8 (12 mg). sub-fr n-ButF
A4 (54–72) was also subjected to CC over silica gel and eluted with an isocratic solvent system [CH2Cl2-MeOH (80:20)] afforded 6 (21 mg).
Fraction n-ButFB (18 g) was subjected to CC over silica gel and eluted with CH2Cl2-MeOH mixture of increasing polarity. 38 new sub-frs of
100 mL each were collected as follows: CH2Cl2-MeOH (90:10)
(sub-frs1–7); CH2Cl2-MeOH (80:20) (sub-frs8–14); CH2Cl2-MeOH (70:30)
(sub-frs15–24); CH2Cl2-MeOH (60:40) (sub-frs25–28); CH2Cl2-MeOH
(50:50) (sub-frs29–36); MeOH (sub-frs37–38). The simple filtration of sub-frs1–3 yielded 5 (9 mg).
Fraction n-ButFC (20 g) was subjected to CC over silica gel and eluted with CH2Cl2-MeOH mixture of increasing polarity. 28 new sub-frs of
100 mL each were collected as follows: CH2Cl2-MeOH (80:20)
(sub-frs1–5); CH2Cl2-MeOH (70:30) (sub-frs6–13); CH2Cl2-MeOH (60:40)
(sub-frs14–19); CH2Cl2-MeOH (50:50) (sub-frs20–26); MeOH
(sub-frs27–28). They were further pooled into 4 other sub-frs according to their TLC profiles: n-ButFC1 (2.1 g; frs1–6); n-ButFC2 (1.8 g; sub-frs7–17); n-ButFC3 (2.6 g; frs18–24) and n-ButFC4 (4.2 g; sub-frs25–28). sub-fr n-ButFA2 (7–17) was subjected to further sephadex LH20 purification with CH2Cl2-MeOH (50:50) to give 7 (12 mg).
2.4. Chemicals
The natural compounds 1–8 (Fig. 1) used in this study were isolated from stem of D. glaucescens as described above. Doxorubicin 98.0% was purchased from Sigma-Aldrich (Munich, Germany) and used as refer-ence drug.
2.5. Cell lines and culture
Four human cancer cell lines and one normal cell line were used in this study. They included A549 human non-small cell lung cancer (NSCLC) cell line, obtained from the Institute for Fermentation, Osaka (IFO, Japan), Caco2 colorectal adenocarcinoma cells obtained from the ŞAP Institute of Turkey (Ankara), HepG2 hepatocarcinoma cells, MCF-7 breast adenocarcinoma cells and the normal CRL2120 human skin fi-broblasts was obtained from ATCC. The cells were maintained as a monolayer in DMEM medium (Sigma-Aldrich, Munich, Germany) me-dium supplemented with 10% fetal calf serum and 1% penicillin (100 U/mL)-streptomycin (100μg/mL) in a humidified 5% CO2atmosphere
at 37 °C.
2.6. Neutral red uptake (NRU) assay
The cytotoxicity of samples was performed by NRU uptake assay as previously described (Borenfreund et al., 1988). This method is based on the ability of viable cells to incorporate and bind the supravital dye
NRU in the lysosomes. The procedure is cheaper and more sensitive than other cytotoxicity tests (Repetto et al., 2008). Samples were added in the culture medium so that dimethylsufoxide (DMSO) used prior for dilution did not exceed 0.1%final concentration. Briefly, cells were detached by treatment with 0.25% trypsin/EDTA (Invitrogen) and an aliquot of 1 × 104cells was placed in each well of a 96-well
cell culture plate (Thermo Scientific, Germany) in a total volume of 200μL. The cells were allowed to attach overnight and subsequently treated with different concentrations of the 12 compounds and doxoru-bicin. Each of the studied samples were immediately added in varying concentrations in additional 100μL of culture medium to obtain a total volume of 200μL/well. After 72 h incubation in humidified 5% CO2atmosphere at 37 °C, the medium was removed and 200μL fresh
medium containing 50μg/mL NRU was added to each well and incuba-tion continued for an addiincuba-tional 3 h at 37 °C in 5% CO2atmosphere. The
dye medium was then removed and each well was then washed rapidly with 200μL phosphate buffer saline (PBS) followed by addition of 200μL of acetic acid–water–ethanol in water (1:49:50). The plates were kept for 15 min at room temperature to extract the dye and then shaken for a few minutes on a GFL 3012 shaker (Gesellschaft für Labortechnik mbH, Burgwedel, Germany). Absorbance was measured on ELx 808 Ultra Microplate Reader (Biotek) equipped with a 540 nm filter. Each assay was done at least three times, with three replicates each. The viability was evaluated based on a comparison with untreated cells. The IC50values represent the sample's concentrations required to
inhibit 50% of cell proliferation and were calculated from a calibration curve by linear regression using Microsoft Excel (Kuete et al., 2011). 2.7. Flow cytometry for cell cycle analysis and detection of apoptotic cells
The cell-cycle analysis was performed byflow cytometry using BD cycletest™ Plus DNA Kit Assay (BD Biosciences, San Jose, USA). The BD Cycletest™ Plus DNA kit provides a set of reagents for isolating and staining cell nuclei. Flow cytometric analysis of differentially stained cells is used to estimate the DNA index (DI) and cell-cycle phase distri-butions. Briefly, MCF-7 cells (3 mL, 1 × 105cells/mL) were seeded into
each well of 6-well plates and allowed to attach for 24 h. The cells which were treated with 1/4 × IC50, 1/2 × IC50and IC50concentrations
of compound 4 and the standard drug, doxorubicin were then grown in 6-well plates for 72 h. The untreated cells (control) were also includ-ed in the assay. They were further trypsinizinclud-ed and suspendinclud-ed in 1 mL PBS, then centrifuged at 400g for 5 min at room temperature (RT). The cells were further processed according to the manufacturer protocol: addition of 250μL of solution A (trypsin buffer), 10 min incubation at RT followed by the addition of 200μL of solution B (trypsin inhibitor and RNase buffer), 10 min incubation at RT followed by the addition of 200μL of solution C (2–8 °C) (propidium iodide stain solution), 10 min on ice. The cells were further measured on a BD FACS Aria I Cell Sorter Flow Cytometer (Becton-Dickinson, Germany). For each sample 104cells were counted. For PI excitation, an argon-ion laser Fig. 1. Chemical structures of the tested compounds. Lanosta-7,24-dien-3-one (1); friedelanone (2); friedelanol (3); 3,3′-di-O-methylellagic acid (4); 3,3′,4′-tri-O-methylellagic acid (5); ellagic acid (6); 3′,4′-di-O-methylellagic acid 4-O-β-D-glucopyranoside (7); 3,3′-di-O-methylellagic acid 4′-O-β-D-xylopyranoside (8).
emitting at 488 nm was used. Cytographs were analyzed using BD FACSDiva™ Flow Cytometry Software Version 6.1.2 (Becton-Dickinson). 2.8. Caspase-Glo 3/7 and caspase-Glo 9 assay
Caspase activity in MCF-7 cells was detected using Caspase-Glo 3/7 and Caspase-Glo 9 Assay kits (Promega, Mannheim, Germany) as previ-ously reported (Kuete et al., 2013a, 2013b, 2014). Cells were treated with compound 4 at their 1/2 × IC50and IC50values with DMSO as
sol-vent control for 6 h. Luminescence was measured using an BioTek Synergy™ HT multi-detection microplate reader. Caspase activity was expressed as percentage of the untreated control.
2.9. Analysis of mitochondrial membrane potential (MMP)
The MMP was analyzed in MCF-7 cells by 5,5 ′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide) (JC-1; Biomol, Hamburg, Germany) staining as previously reported (Kuete et al.,
2013a, 2013b, 2014). Cells (3 mL, 1 × 105cells/mL) treated for 72 h
with different concentrations (1/4 × IC50, 1/2 × IC50and IC50) of
com-pound 4 and doxorubicin (drug control) or DMSO (solvent control) were incubated with JC-1 staining solution for 30 min according to the manufacturer's protocol as reported earlier. Subsequently, cells were measured in a BD FACS Aria I Cell Sorter Flow Cytometer (Becton-Dick-inson, Germany). The JC-1 signal was measured at an excitation of 561 nm (150 mW) and detected using a 586/15 nm band-passfilter. The signal was analyzed at 640 nm excitation (40 mW) and detected using a 730/45 nm bandpassfilter. Cytographs were analyzed using BD FACSDiva™ Flow Cytometry Software Version 6.1.2 (Becton-Dickin-son). All experiments were performed at least in triplicate.
2.10. Measurement of reactive oxygen species (ROS)
The 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFH-DA)
(Sigma-Aldrich) was used for the detection of ROS in MCF-7 cells treat-ed with compound 4 and doxorubicin (drug control) or DMSO (solvent control) using OxiSelect™ Intracellular ROS Assay Kit (Green
Fluorescence) as recommended by the manufacturer, Cell Biolabs Inc. (San Diego, USA). This is a cell-based assay for measuring hydroxyl, peroxyl, or other reactive oxygen species activity within a cell. The assay employs the cell-permeable fluorogenic probe 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA). DCFH-DA is diffused into cells and is deacetylated by cellular esterases to non-fluorescent 2′,7′-dichlorodihydrofluorescin (DCFH), which is rapidly oxidized to highly fluorescent 2′,7′-dichlorofluorescein (DCF) by ROS. Cells (1 × 104cells) were treated with samples at 1/4 × IC
50, 1/2 × IC50and
IC50for 24 h. After addition of 100μL 1X DCFH-DA/DMEM solution to
cells and incubation at 37 °C for 30–60 min, the fluorescence was mea-sured using SpectraMax® M5 Microplate Reader (Molecular Devices, Biberach, Germany) at 480/530 nm. All experiments were performed at least in triplicate.
3. Results and discussion
Compounds isolated from the stem of Desbordesia glaucescens were elucidated using physical and NMR data and comparison with literature (Data available in supporting information). They included three triterpenoids namely lanosta-7,24-dien-3-one C30H48O (1; m/z: 424)
(Nana et al., 2012), friedelanone C30H50O (2; m/z: 426; m.p.
264–265 °C) (Mahato and Kundu, 1994) and friedelanol C30H52O (3;
m/z: 428; m.p. 288–289 °C) (Kamperdick et al., 1997) andfive ellagic acid derivatives: 3,3′-di-O-methylellagic acid C16H10O8(4; m/z = 330)
(Ye et al., 2007), 3,3′,4′-tri-O-methylellagic acid C17H12O8(5; m/z:
344; m.p. 287–289 °C) (Ye et al., 2007), ellagic acid C14H6O8(6; m/z:
330) (Li et al., 1999), 3′,4′-di-O-methylellagic acid 4-O-β-D -glucopyranoside C22H20O13 (7; m/z: 492; m.p. 245 et 247 °C)
(DongmoMafodong et al., 2015) and 3,3′-di-O-methylellagic acid
4′-O-β-D-xylopyranoside C21H18O12(8; m/z: 462) (Liu et al., 2014).
Com-pound 7 as well as a novel ellagic acid derivative, desglauside, and oleanolic acid andβ-sitosterol-3-O-β-D-glucopyranoside were previ-ously isolated from the leaves of D. glaucescens (DongmoMafodong et al., 2015).
The cytotoxicity of the 8 isolated compounds and doxorubicin was determined by the NRU uptake assay and the recorded IC50values are
3.1 62.8 15.2 12.8 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Control G row th (pe rc e nt a g e of c o nt ro l) G2/M S G0/G1 Sub-G0 27.6 47.8 60 27.8 16.7 11.5 12.9 10.1 7.6 12.5 11.8 12.8 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100%
1/4xIC50 1/2xIC50 IC50
G row th (pe rc e nt a g e of c o nt ro l) Doxorubicin G2/M S G0/G1 Sub-G0 23.5 28.6 31.8 38.3 37 34.3 15.6 13.7 12.4 11.6 9.8 9 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100%
1/4xIC50 1/2xIC50 IC50
G row th (pe rc e nt a g e of c o nt ro l) Compound 4 G2/M S G0/G1 Sub-G0
Fig. 2. Effects 3,3′-di-O-methylellagic acid (4) and doxorubicin on cell cycle distribution in MCF-7 cells. IC50values were 11.23μM (4) and 0.35 μM (doxorubicin). Table 1
Cytotoxicity of tested compounds and doxorubicin towards cancer cell lines and normal cells as determined by the neutral red assay. Compounds Cell lines, IC50values (inμM) and selectivity index* (in bracket)
A549 Caco-2 MCF-7 HepG2 CRL2120
1 82.43 ± 6.42 (N1.15) N94.39 N94.39 43.63 ± 3.17 (N2.16) N94.39 2 N93.90 82.39 ± 7.02 (N1.14) 60.61 ± 4.39 (N1.54) N93.90 N93.90 3 80.79 ± 5.69 (N1.16) 35.37 ± 2.18 (N2.64) N93.46 22.24 ± 2.01 (N4.20) N93.46 4 31.70 ± 1.76 (N3.84) 44.65 ± 4.35 (N2.72) 11.23 ± 0.17 (N10.83) 13.47 ± 0.55 (N9.02) N121.58 5 N116.28 N116.28 N116.28 55.44 ± 3.91 (N2.10) N116.28 6 77.73 ± 5.96 (N1.72) 74.83 ± 6.22 (N1.78) 14.07 ± 0.88 (N9.48) 22.17 ± 1.37 (N6.01) N133.33 7 N81.30 N81.30 N81.30 57.95 ± 3.16 (N1.40) N81.30 8 N86.58 N86.58 N86.58 N86.58 N86.58 Doxorubicin 1.01 ± 0.19 0.72 ± 0.13 0.35 ± 0.05 0.18 ± 0.03 0.59 ± 0.01
(*): The selectivity index was determined as the ratio of IC50value in the CRL2120 normalfibroblasts divided by the IC50in the cancer cell lines. In bold: significant activity (Boik, 2001;
Brahemi et al., 2010; Kuete and Efferth, 2010, 2015); lanosta-7,24-dien-3-one (1); friedelanone (2); friedelanol (3), 3,3′-di-O-methylellagic acid (4), 3,3′,4′-tri-O-methylellagic acid (5),
summarized inTable 1. The selectivity index was determined as the ratio of IC50value in the CRL2120 normalfibroblast divided by the IC50
in the cancer cell line. Compounds 4 and 6 as well as doxorubicin had IC50values below 45μM in the four tested cancer cell lines meanwhile
other compounds displayed selective activity. The obtained IC50values
ranged from 11.23μM (towards breast adenocarcinoma MCF-7 cells) to 44.65μM (colon carcinoma Caco-2 cells) for 4, from 14.07 μM (to-wards MCF-7 cells) to 77.73μM (Caco-2 cells) for 6 and from 0.07 μM (towards SPC212 cells) to 1.01μM (A549 cells) for doxorubicin. The two most active compounds (4 and 6) were generally less toxic towards normal CRL2120fibroblast than carcinoma cells, and the obtained selec-tivity indexes were above 1.00 in the majority of the cases (Table 1). Compound 4 having the lowest IC50values than other compounds as
well as doxorubicin were further tested for the effects on cell cycle dis-tribution, caspases activity, MMP loss and ROS production in MCF-7 cells.
Compound 4 was analyzed for its ability to alter the distribution of the cell cycle of MCF-7 breast cancer cells (Fig. 2). It was observed that this phytochemical induced concentration-dependent cell cycle modi fi-cations with progressive increase of sub-G0/G1 phase cells. It induced cell cycle arrest in S phases. MCF-7 cells treated with the compound 4 progressively underwent apoptosis, with increase of sub-G0/G1 cells from 27.6% (1/4 IC50) to 60% (IC50) whilst doxorubicin also caused up
to 60% sub-Go/G1 phase with IC50treatment in comparison to only
3.1% in non-treated cells. Upon treatment of MCF-7 cells with equiva-lent (eq.) to the 1/2 × IC50and IC50for 6 h, compound 4 did not activate
caspase 3/7 and caspase 9 contrary to doxorubicin (Fig. 3). The integrity of the MMP was also investigated upon treatment of MCF-7 cells with
Fig. 4. Effects of 3,3′-di-O-methylellagic acid (4) and doxorubicin on MMP in MCF-7 cells for 72 h. IC50values were 11.23μM (4) and 0.35 μM (doxorubicin). Cells were treated with 1/ 4 × IC50(C1), 1/2 × IC50(C2) and IC50(C3) of each compound.
0 0.5 1 1.5 2 2.5
½×IC(50) IC(50) ½×IC(50) IC(50) Compound 4 Doxorubicin Fol d i n c re a s e caspase 3/7 caspase 9
Fig. 3. Effects of 3,3′-di-O-methylellagic acid (4) and doxorubicin on the activation caspases 3/7 and 9 in MCF-7 cells. IC50values were 11.23μM (4) and 0.35 μM (doxorubicin).
compound 4 and doxorubicin. Treatment with compound 4 at eq. to the 1/4 × IC50, 1/2 × IC50and IC50values for 72 h induced
concentration-dependent depletion of MMP (Fig. 4). The percentage loss of MMP ranged from 9.9% (1/4 × IC50) to 16.5% (IC50). In similar experimental
condition, doxorubicin caused 26% loss of MMP meanwhile only 4.3% was observed with non-treated control. After treatment of MCF-7 cells with compound 4 and doxorubicin at eq. to the 1/4 × IC50, 1/2 × IC50
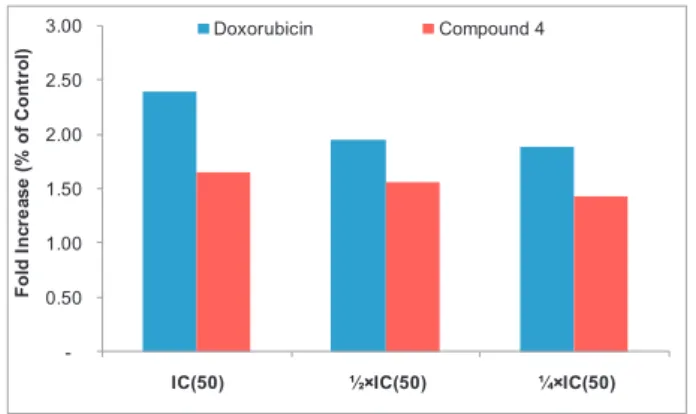
and IC50values for 24 h, the production of ROS in cells was also analyzed
(Fig. 5). Compound 4 slightly induced increased ROS levels (less than
2-fold (at IC50) as compared with non-treated cells meanwhile the
in-crease was more than 2-fold after treatment with doxorubicin. In regards to the high incidence of cancer globally, there is a contin-ued need for new alternatives for treatment, and natural products play an important role in the discovery of cytotoxic compounds (George et al., 2010; Kuete and Efferth, 2011; Dzoyem et al., 2012; Kuete and
Efferth, 2015). In the present work, compounds isolated from
Desbordesia glaucescens were tested for their cytotoxic effects against various carcinoma cell lines, including breast, colon, lung and liver can-cers. These cancer types are amongst the leading cause of cancer death worldwide (Torre et al., 2015). According to the National Cancer Insti-tute (NCI), phytochemicals having IC50 values around or below
4μg/mL or 10 μM (Boik, 2001; Brahemi et al., 2010; Kuete and Efferth, 2015) can be considered as potential cytotoxic substances. In the pres-ent study IC50values obtained were all above 10μM; however, the
IC50values of 11.23μM and 13.47 μM obtained with compound 4 against
breast adenocarcinoma MCF-7 cells and hepatocarcinoma HepG2 re-spectively, were not far from this set bar. This suggests that this com-pound could be explored more to develop a possible cytotoxic drug. Possible modification of the chemical structure of both compounds 4 and 6 could be envisaged to improve their activity. Importantly, the se-lectivity indexes obtained with the two compounds were above 1, indi-cating that they were more toxic towards carcinoma cells than towards normal CRL2120fibroblast in all the cases. The cytotoxicity of the ellagic acid derivatives 4 and 6 are in accordance with previous studies; in fact, ellagic acid has been proposed as a promising candidate for therapeutic use in colon cancer (Yousef et al., 2016). In this study, this compound was active towards Caco-2 colon adenocarcinoma, though the effect was rather low (IC50of 74.83μM). An isomer of compound 4 namely
4,4′-di-O-methylellagic acid also efficiently inhibits colon cancer cell growth through a mechanism involving WNT16 (Ramirez de Molina
et al., 2015), confirming the role of ellagic acid as potential cytotoxic
drugs. In this work we provided additional information on the cytotox-icity of this compound on breast, lung and liver cancers. Compound 4 in-duces apoptosis in MCF-7 cells, mediated by alteration on MMP. Ellagic acid was also found to induce apoptosis via depolarization of mitochon-dria in human neuroblastoma cells (Alfredsson et al., 2014). It has been reported that the breast cancer cell line MCF-7 lacks a functional caspase-3 gene product (Jänicke et al., 1998)). This could explained
the lack of induction of apoptosis by compound 4 in MCF-7 cells as ob-served in the present study.
Regarding the structure–activity relationship, it appears that both terpenoids and phenolics isolated from Desbordesia glaucescens had cy-totoxic effects. The best activities were obtained with ellagic acid (6) and its derivative 4. Within terpenoids, friedelanol is more active than friedelanone on Caco-2, MCF-7 and HepG2 cells; the presence of hy-droxyl (−OH) group at C-3 influences for the anticancer activity. With-in phenolics, the 3,3′-di-O-méthylellagic acid is more active than the 3,3′,4′-tri-O-méthylellagic acid on A549, Caco-2, MCF-7 and HepG2 cells; hence, the–OH group at C-4 also seems to influence the cytotoxic activity ellagic acid derivatives.
4. Conclusions
In the present study, we demonstrated the cytotoxicity of com-pounds isolated from Desbordesia glaucescens against human carcinoma cell lines. Ellagic acid (6) and its derivative 4 displayed cytotoxic effects on all tested cancer cell lines. Compound 4 induced apoptosis in MCF-7 cells mediated by MMP loss. The studied compounds and especially 4 and 6 deserve more investigations to develop novel cytotoxic drugs against cancers. Abbreviations 1 lanosta-7,24-dien-3-one 2 friedelanone 3 friedelanol 4 3,3′-di-O-methylellagic acid 5 3,3′,4′-tri-O-methylellagic acid 6 ellagic acid
7 3′,4′-di-O-methylellagic acid 4-O-β-D-glucopyranoside 8 3,3′-di-O-methylellagic acid 4′-O-β-D-xylopyranoside
DMSO dimethylsufoxide
H2DCF 2′,7′-Dichlorodihydrofluorescein
H2DCFH-DA 2′,7′-Dichlorodihydrofluorescein diacetate
MMP mitochondrial membrane potential NRU neutral red uptake
PBS phosphate buffer saline ROS reactive oxygen species
Acknowledgments
V.K. and H.S. are thankful to Scientific and Technological Research Counsel of Turkey (TÜBİTAK) for 6 months travel grant (to V.K.) and to Scientific Research Projects Commission of Anadolu University, Eski-sehir, Turkey for the funding grant 1507F563 (to V.K. and H.S.). A grant for part of this work was also provided by International Science Pro-gramme, Uppsala University, Sweden (ISP)-KEN-02 project. IC would like to thank the Scientific Research Projects Commission of Anadolu University, Eskisehir, Turkey for the funding grant (1306F110). Authors are also thankful toŞennur Görgülü for FACS measurements.
Appendix A. Supplementary data
Supplementary data to this article can be found online athttp://dx.
doi.org/10.1016/j.sajb.2017.03.031.
References
Alfredsson, C.F., Ding, M., Liang, Q.L., Sundstrom, B.E., Nanberg, E., 2014.Ellagic acid in-duces a dose- and time-dependent depolarization of mitochondria and activation of caspase-9 and -3 in human neuroblastoma cells. Biomedicine & Pharmacotherapy 68, 129–135.
Boik, J., 2001.Natural Compounds in Cancer Therapy. Oregon Medical Press, Minnesota USA. -0.50 1.00 1.50 2.00 2.50 3.00
IC(50) ½×IC(50) ¼×IC(50)
Fol d I n c re a s e (% of C o nt ro l) Doxorubicin Compound 4
Fig. 5. Induction of ROS in MCF-7 cells after treatment with 3,3′-di-O-methylellagic acid (4) and doxorubicin for 24 h. IC50values were 11.23μM (4) and 0.35 μM (doxorubicin).
Borenfreund, E., Babich, H., Martin-Alguacil, N., 1988.Comparisons of two in vitro cytotox-icity assays—the neutral red (NR) and tetrazolium MTT tests. Toxicology In Vitro 2, 1–6.
Brahemi, G., Kona, F.R., Fiasella, A., Buac, D., Soukupova, J., Brancale, A., Burger, A.M., Westwell, A.D., 2010.Exploring the structural requirements for inhibition of the ubiq-uitin E3 ligase breast cancer associated protein 2 (BCA2) as a treatment for breast cancer. Journal of Medicinal Chemistry 53, 2757–2765.
Bray, F., Ren, J.S., Masuyer, E., Ferlay, J., 2013.Global estimates of cancer prevalence for 27 sites in the adult population in 2008. International Journal of Cancer 132, 1133–1145. DongmoMafodong, F.L., Tsopmo, A., Awouafack, M.D., Roland, T.T., Dzoyem, J.P., Tane, P., 2015.A novel ellagic acid derivative from Desbordesia glaucescens. Natural Product Communications 10, 1709–1710.
Dzoyem, J.P., Nkuete, A.H., Kuete, V., Tala, M.F., Wabo, H.K., Guru, S.K., Rajput, V.S., Sharma, A., Tane, P., Khan, I.A., Saxena, A.K., Laatsch, H., Tan, N.H., 2012.Cytotoxicity and anti-microbial activity of the methanol extract and compounds from Polygonum limbatum. Planta Medica 78, 787–792.
Fadeyi, S.A., Fadeyi, O.O., Adejumo, A.A., Okoro, C., Myles, E.L., 2013.In vitro anticancer screening of 24 locally used Nigerian medicinal plants. BMC Complementary and Al-ternative Medicine 13, 79.
Ferlay, J., Soerjomataram, I., Ervik, M., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D.M., Forman, D., Bray, F., 2013. International Agency for Research on Cancer. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 (globocan.iarc.frAccessed December 12, 2013).
George, S., Bhalerao, S.V., Lidstone, E.A., Ahmad, I.S., Abbasi, A., Cunningham, B.T., Watkin, K.L., 2010.Cytotoxicity screening of Bangladeshi medicinal plant extracts on pancre-atic cancer cells. BMC Complementary and Alternative Medicine 10, 1–11. Ipek, E., Zeytinoglu, H., Okay, S., Tuylu, B.A., Kurkcuoglu, M., Baser, K.H.C., 2005.
Genotoxicity and antigenotoxicity of Origanum oil and carvacrol evaluated by Ames salmonella/microsomal test. Food Chemistry 93, 551–556.
Jänicke, R.U., Sprengart, M.L., Wati, M.R., Porter, A.G., 1998.Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. Journal of Bio-logical Chemistry 273, 9357–9360.
Kamperdick, C., Adam, G., Van, N.H., Sung, T.V., 1997.Chemical constituents of Madhuca pasquiery. Zeitschrift für Naturforschung 52c, 295–300.
Kuete, V., Efferth, T., 2010.Cameroonian medicinal plants: pharmacology and derived natural products. Frontiers in Pharmacology 1, 123.
Kuete, V., Efferth, T., 2011.Pharmacogenomics of Cameroonian traditional herbal medi-cine for cancer therapy. Journal of Ethnopharmacology 137, 752–766.
Kuete, V., Efferth, T., 2015.Africanflora has the potential to fight multidrug resistance of cancer. BioMed Research International 2015, 914813.
Kuete, V., Krusche, B., Youns, M., Voukeng, I., Fankam, A.G., Tankeo, S., Lacmata, S., Efferth, T., 2011.Cytotoxicity of some Cameroonian spices and selected medicinal plant ex-tracts. Journal of Ethnopharmacology 134, 803–812.
Kuete, V., Fankam, A.G., Wiench, B., Efferth, T., 2013a.Cytotoxicity and modes of action of the methanol extracts of six Cameroonian medicinal plants against
multidrug-mesistant tumor cells. Evidence-Based Complementary and Alternative Medicine 2013, 285903.
Kuete, V., Sandjo, L., Nantchouang Ouete, J., Fouotsa, H., Wiench, B., Efferth, T., 2013b. Cy-totoxicity and modes of action of three naturally occuring xanthones (8-hydroxycudraxanthone G, morusignin I and cudraxanthone I) against sensitive and multidrug-resistant cancer cell lines. Phytomedicine 21, 315–322.
Kuete, V., Tankeo, S.B., Saeed, M.E., Wiench, B., Tane, P., Efferth, T., 2014.Cytotoxicity and modes of action offive Cameroonian medicinal plants against multi-factorial drug re-sistance of tumor cells. Journal of Ethnopharmacology 153, 207–219.
Letouzey, R., 1982.Manuel de botanique forestière Afrique tropicale, botanique générale. GERDAT-CTFT, France (Nogent-sur-Marne).
Li, X.-C., Elsohly, H.N., Hufford, C.D., Clark, A.M., 1999.NMR assignments of ellagic acid de-rivatives. Magnetic Resonance in Chemistry 37, 856–859.
Liu, Q., Lu, D., Jin, H., Yan, Z., Li, X., Yang, X., Guo, H., Qin, B., 2014.Allelochemicals in the rhizosphere soil of Euphorbia himalayensis. Journal of Agricultural and Food Chemis-try 62, 8555–8561.
Mahato, S.B., Kundu, S.P., 1994.13C NMR spectra of pentacyclic triterpenoids—a compila-tion and some salient features. Phytochemistry 37, 1517–1575.
Nana, F., Sandjo, L.P., Keumedjio, F., Ambassa, P., Malik, R., Kuete, V., Rincheval, V., Choudhary, M.I., Ngadjui, B.T., 2012.Ceramides and cytotoxic constituents from Ficus glumosa Del. (Moraceae). Journal of the Brazilian Chemical Society 23, 1–9. Ramirez de Molina, A., Vargas, T., Molina, S., Sanchez, J., Martinez-Romero, J.,
Gonzalez-Vallinas, M., Martin-Hernandez, R., Sanchez-Martinez, R., Gomez de Cedron, M., Davalos, A., Calani, L., Del Rio, D., Gonzalez-Sarrias, A., Espin, J.C., Tomas-Barberan, F.A., Reglero, G., 2015.The ellagic acid derivative 4,4′-di-O-methylellagic acid effi-ciently inhibits colon cancer cell growth through a mechanism involving WNT16. Journal of Pharmacology and Experimental Therapeutics 353, 433–444.
Repetto, G., del Peso, A., Zurita, J.L., 2008.Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nature Protocols 3, 1125–1131.
Torre, L.A., Bray, F., Siegel, R.L., Ferlay, J., Lortet-Tieulent, J., Jemal, A., 2015.Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians 65, 87–108.
Unlu, M., Ergene, E., Unlu, G.V., Zeytinoglu, H.S., Vural, N., 2010.Composition, antimicro-bial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food and Chemical Toxicology 48, 3274–3280.
Ye, G., Peng, H., Fan, M., Huang, C.-G., 2007.Ellagic acid derivatives from the stem bark of Dipentodon sinicus. Chemistry of Natural Compounds 43, 125–127.
Yousef, A.I., El-Masry, O.S., Yassin, E.H., 2016.The anti-oncogenic influence of ellagic acid on colon cancer cells in leptin-enriched microenvironment. Tumor Biology 1–9. Zeytinoglu, H.,İncesu, Z., Baser, K.H.C., 2003.Inhibition of DNA synthesis by carvacrol in
mouse myoblast cells bearing a human N-ras oncogene. Phytomedicine 10, 292–299. Zeytinoglu, H., Incesu, Z., AyazTuylu, B., Turk, A.O., Barutca, B., 2008.Determination of genotoxic, antigenotoxic and cytotoxic potential of the extract from lichen Cetrariaaculeata (Schreb.) Fr. in vitro. Phytotherapy Research 22, 118–123.