Copyright © 2016 Wolters Kluwer Health, Inc. All rights reserved.
Bioprostethic mitral valve thrombosis due to oral
contraceptive drug use and management with ultra-slow
thrombolytic therapy
Mahmut Yesin
a, Macit Kalc¸ik
b, Sabahattin Gu¨ndu¨z
c, Mehmet Ali Astarciog˘lu
d,
Mustafa Ozan Gu¨rsoy
e, Su¨leyman Karakoyun
fand Mehmet O
¨ zkan
c,gProsthetic valve thrombosis is a severe complication, which usually occurs in inadequately anticoagulated patients. Mechanical valve thrombosis is more common than bioprosthetic valve thrombosis (BVT). Oral
contraceptive drugs are associated with increased risk of thromboembolism in women. The possible association between oral contraceptive drug use and BVT has never been reported before. We present a case of obstructive BVT occurring after the use of an oral contraceptive drug and successful management with ultra-slow
thrombolytic therapy.Blood Coagul Fibrinolysis 27:220–222 Copyright ß 2016 Wolters Kluwer Health, Inc. All rights reserved.
Blood Coagulation and Fibrinolysis2016, 27:220–222
Keywords: bioprosthetic valve, oral contraceptive drugs, thrombolytic therapy, thrombosis, tissue-type plasminogen activator
a
Department of Cardiology, Kars Karakani State Hospital, Kars,b
Department of Cardiology, I˙skilip Atıf Hoca State Hospital, C¸ orum,c
Department of Cardiology, Kosuyolu Kartal Heart Training and Research Hospital, Istanbul,d
Department of Cardiology, Evliya C¸ elebi Training and Research Hospital, Ku¨tahya,e
Department of Cardiology, Gaziemir State Hospital, I˙zmir,f
Department of Cardiology, Kars Kafkas University, Faculty of Medicine, Kars andg
Division of Health Sciences, Ardahan University, Ardahan, Turkey
Correspondence to Mahmut Yesin, MD, Ug˘urmumcu Mah., Fatih Sultan Mehmet Caddesi Serc¸e Sokak No: 33 Daire: 14 Kartal, Istanbul, Turkey
Tel: +90 532 4726596; fax: +90 216 4596321; e-mail: mahmutyesin@yahoo.com
Received22 June 2015 Revised 29 July 2015 Accepted13 August 2015
Introduction
Prosthetic valve thrombosis (PVT) is a potentially life-threatening complication associated with high morbidity and mortality [1]. The incidence of left-sided PVT is reported to be 0.5–8% per patient-year [2]. Inadequate anticoagulation, atrial fibrillation, left atrial enlargement, multiple valve replacement, ventricular dysfunction, and pregnancy are the major risk factors for the development of PVT [3,5]. Transesophageal echocardiography (TEE) plays a key role in the diagnosis. The treatment options are surgery, thrombolytic therapy, and intravenous unfractionated heparin; however, the optimal manage-ment strategy is still controversial. We herein present a case of mitral bioprosthetic valve thrombosis (BVT) which was diagnosed with TEE and successfully treated with thrombolytic therapy.
Case report
A 44-year-old woman who had undergone bioprostethic mitral valve replacement 6 years earlier was admitted to our hospital with New York Health Association functional class 4 dyspnea. Her blood pressure was 110/65 mmHg and electrocardiogram showed normal sinus rhythm. She had given up warfarin 6 months after surgery and had been treated with acetylsalicylic acid (100 mg/day) thereafter. She was nondiabetic, nonhypertensive but a current smo-ker. She had started to take an oral contraceptive drug including ethinyl estradiol and desogestrel 1 month before admission. Transthoracic echocardiography (TTE) and subsequently TEE were performed on admission for
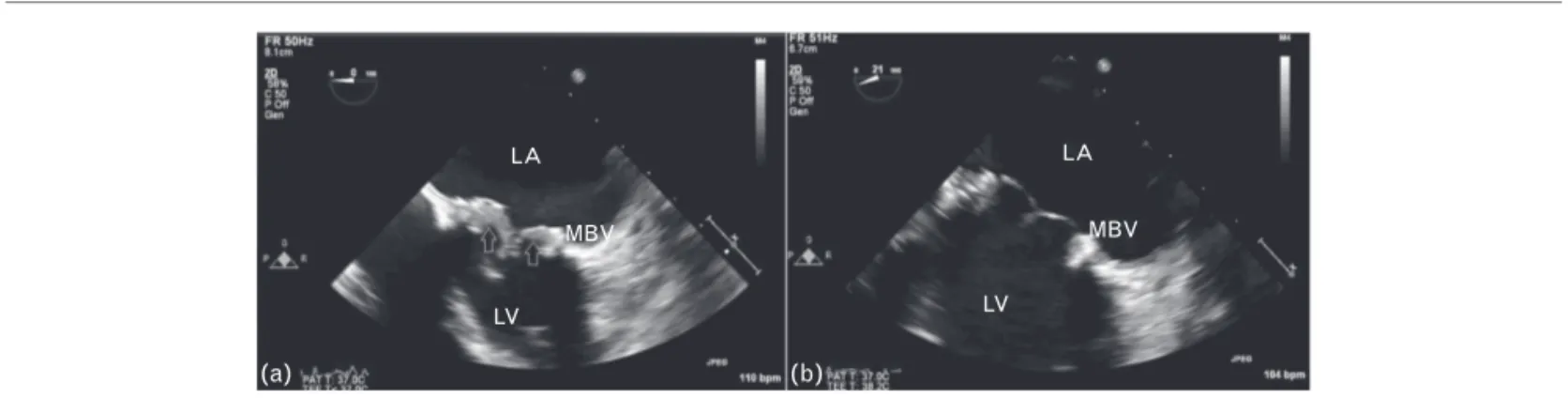
the evaluation of prosthetic mitral valve. TTE showed normal left ventricular ejection fraction and TEE revealed increased mean transvalvular gradient of 24 mmHg, decreased valve area of 1.6 cm2(Fig. 1a) caused by a large BVT (Fig. 2a and see supplementary Video 1, http:// links.lww.com/BCF/A22). Thrombolytic therapy with low-dose (25 mg) and ultra-slow infusion (25 h) of tissue plasminogen activator (t-PA) without bolus under the guidance of serial TEE was planned. The obstruction signs still continued after the first thrombolytic therapy session. After the second thrombolytic therapy session normalization of mean gradient (5 mmHg) and valve area (2.3 cm2) with complete lysis of the thrombus was observed without any complication (Figs. 1b and 2b and see supple-mentary video 2, http://links.lww.com/BCF/A23). The approximate time point of breakdown of the thrombus was 50 h after the initial t-PA dose.
The patient was investigated for possible genetic throm-bophilic conditions. In order to determine the genetic risk factors for thrombophilia, she was evaluated for factor V G1691A (Leiden), factor V H1299R (R2), prothrombin G20210A factor XIII V34L, b fibrinogen 455 G-A, plas-minogen activator inhibitor-1 (PAI) 4G-5G, glycoprotein IIIa (GPIIIa) L33P (HPA-1), methylentetrahydrofolate reductase (MTHFR) C677T, MTHFR A1298C, angio-tensin-converting enzyme (ACE) gene mutations found in our cardiovascular panel. Protein C and protein S deficiencies were also evaluated. There was no genetic mutation or protein deficiency which may cause a hypercoagulable state. Subsequently, oral contraceptive 220 Case report
0957-5235 Copyright ß 2016 Wolters Kluwer Health, Inc. All rights reserved. DOI:10.1097/MBC.0000000000000420 Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.bloodcoagulation.com).
Copyright © 2016 Wolters Kluwer Health, Inc. All rights reserved.
medication was discontinued and the patient was dis-charged with 100 mg acetylsalicylic acid medication.
Discussion
PVT is a severe complication after heart valve replace-ment surgery, which should be suspected in patients with exertional dyspnea and signs of cerebrovascular embo-lism. There is an increased risk of PVT in patients with risk factors such as atrial fibrillation, left atrial enlarge-ment, multiple valve replaceenlarge-ment, and ventricular dys-function. The most common cause of PVT is inadequate anticoagulant therapy in up to 82% of cases [2]. Several genetic mutations and protein C or protein S deficiencies may create a thrombophilic condition; however, in the present case, the hypercoagulable state caused by oral contraceptive drug use was thought to be the only risk factor for PVT development.
Although PVT is observed more commonly with mech-anical prostheses, it is also a serious complication for bioprosthetic valves especially in postoperative 3 months with an incidence of 6% among patients with suspected bioprosthetic valve dysfunction [6].
Oral contraceptive drugs may contain different types of progestogens including lynestrenol (firstgeneration pro-gestogen), levonorgestrel or norgestrel (second-gener-ation progestogens), and desogestrel or gestodene (third-generation progestogens). The associations of oral contraceptive drug use with pulmonary embolism [7] and cerebral venous sinus thrombosis [8] have been reported previously. Although mechanical PVT has been rarely reported in several patients using oral contraceptive drugs, BVT is first to be reported in the current literature. Obstructive BVT patients are usually admitted with pul-monary edema, cardogenic shock, systemic thromboem-bolism, or arrhythmia. Although TTE can evaluate leaflet motion, transvalvular gradients, and prosthetic valve area in patients with suspected BVT, TEE provides valuable data regarding BVT and left atrial body or appendage thrombosis and guides the treatment strategies.
Treatment modalities for PVT may include heparin treatment, thrombolytic therapy, and surgery. Guidelines lack definitive class I recommendations due to lack of randomized controlled trials, and usually leave the choice of treatment to the clinician’s experience. Surgery is A rare cause of bioprostethic valve thrombosisYesin et al. 221
Fig. 1
(a) (b)
Transesophageal echocardiography revealed increased mean transvalvular gradient of 24 mmHg with decreased valve area of 1.6 cm2on admission (a) and normalization of mean gradient (5 mmHg) and valve area (2.3 cm2) after thrombolytic treatment (b).
Fig. 2 LA MBV LV LA MBV LV (a) (b)
Transesophageal echocardiography revealed an obstructive bioprostethic valve thrombosis on admission (a) and complete lysis of the thrombus after thrombolytic treatment (b).
Copyright © 2016 Wolters Kluwer Health, Inc. All rights reserved.
suggested as a first-line strategy in most situations of left sided PVT; however, thrombolytic therapy has been recently used with successful outcomes [4–10]. We have previously reported that low-dose (25 mg) and slow infu-sion (6 h) of t-PA is very safe and associated with very high thrombolytic success in this regard [9]. Moreover, in a recent study we have suggested that further pro-longation of the tPA regimen with low-dose (25 mg) and ultra-slow (25 h) infusion could be associated with lower complication rates without compromising the over-all success [10].
Conclusion
Oral contraceptive drug use may increase the risk of thrombosis in women and should be used cautiously in patients with prosthetic heart valves. Thrombolytic therapy with low-dose (25 mg), ultra-slow (25 h) infusion of t-PA may be an effective and safe treatment option in patients with BVT.
Acknowledgements
All of the authors contributed planning, conduct, and reporting of the work and are responsible for the overall content as guarantors.
Conflicts of interest
There are no conflicts of interest.
References
1 Keuleers S, Herijgers P, Herregods MC, Budts W, Dubois C, Meuris B, et al. Comparison of thrombolysis versus surgery as a first line therapy for prosthetic heart valve thrombosis. Am J Cardiol 2011; 107:275e279. 2 Lengyel M. Diagnosis and treatment of left-sided prosthetic valve
thrombosis. Expert Rev Cardiovasc Ther 2008; 6:85e93.
3 Laplace G, Lafitte S, Labe`que JN, Perron JM, Baudet E, Deville C, et al. Clinical significance of early thrombosis after prosthetic mitral valve replacement: a postoperative monocentric study of 680 patients. J Am Coll Cardiol 2004; 43:1283e1290.
4 Ca´ceres-Lo´riga FM, Pe´rez-Lo´pez H, Morlans-Herna´ndez K, Facundo-Sa´nchez H, Santos-Gracia J, Valiente-Mustelier J, et al. Thrombolysis as first choice therapy in prosthetic heart valve thrombosis. A study of 68 patients. J Thromb Thrombolysis 2006; 21:185e190.
5 Ozkan M, Cakal B, Karakoyun S, Gu¨rsoy OM, Cevik C, Kalc¸ik M, et al. Thrombolytic therapy for the treatment of prosthetic heart valve thrombosis in pregnancy with low-dose, slow infusion of tissue-type plasminogen activator. Circulation 2013; 128:532e540.
6 Oliver JM, Galloge P, Gonzalez A, Dominguez FJ, Gamallo C, Mesa JM. Bioprosthetic mitral valve thrombosis: clinical profile, transesophageal echocardiographic features, and follow-up after anticoagulant therapy. J Am Soc Echocardiogr 1996; 9:691–699.
7 Jordan WM. Pulmonary embolism. Lancet 1961; 2:1146–1147. 8 Amoozegar F, Ronksley PE, Sauve R, Menon BK. Hormonal contraceptives
and cerebral venous thrombosis risk: a systematic review and meta-analysis. Front Neurol 2015; 6:7.
9 O¨ zkan M, Gunduz S, Biteker M, Astarciog˘lu MA, C¸evik C, Kaynak E, et al. Comparison of different TEE-guided thrombolytic regimens for prosthetic valve thrombosis: the TROIA trial. JACC Cardiovasc Imaging 2013; 6:206–216.
10 O¨ zkan M, Gu¨ndu¨z S, Gu¨rsoy MO, Karakoyun S, Astarciog˘lu MA, Kalc¸ik M, et al. Ultra-slow thrombolytic therapy: A novel strategy in the management of PROsthetic MEchanical valve Thrombosis and the prEdictors of outcomE: the ultra-slow PROMETEE trial. Am Heart J 2015; 170:409 – 418.