Full Terms & Conditions of access and use can be found at
https://www.tandfonline.com/action/journalInformation?journalCode=upst20
Particulate Science and Technology
An International Journal
ISSN: 0272-6351 (Print) 1548-0046 (Online) Journal homepage: https://www.tandfonline.com/loi/upst20
The effect of single and combined coagulation/
flocculation methods on the sedimentation
behavior and conductivity of bentonite
suspensions with different swelling potentials
V. Onen & M. Gocer
To cite this article: V. Onen & M. Gocer (2019) The effect of single and combined coagulation/ flocculation methods on the sedimentation behavior and conductivity of bentonite suspensions with different swelling potentials, Particulate Science and Technology, 37:7, 827-834, DOI: 10.1080/02726351.2018.1454993
To link to this article: https://doi.org/10.1080/02726351.2018.1454993
Published online: 10 May 2018.
Submit your article to this journal
Article views: 145
View related articles
The effect of single and combined coagulation/flocculation methods on the
sedimentation behavior and conductivity of bentonite suspensions
with different swelling potentials
V. Onen and M. GocerDepartment of Mining Engineering, Selcuk University, Konya, Turkey
ABSTRACT
In this study, the sedimentation behavior of bentonite (Na and Ca bentonite) suspensions with different swelling potentials was investigated with single and combined coagulation and flocculation methods. The samples exhibited a negative surface charge over a broad pH range and had a relatively high suspension stability. Al2(SO4)3, FeCl3, MgCl2, CaCl2, and NaCl were used as coagulants. All coagulants have
provided sedimentation efficiencies higher than 85% with Ca bentonite suspension, but only 22% efficiency was seen with Na bentonite suspensions. The effectiveness of coagulants increased with higher ionic values of the metal salts. Versus monovalent cations, multivalent cations had a greater influence on the zeta potential of the samples. Higher coagulant concentrations enhance the conductivities of the suspensions. In flocculation, anionic (A-150), cationic (C-521) and nonionic (N-100) flocculants were used. For a Ca bentonite suspension, all flocculants have 98% efficiency. The anionic flocculant was more efficient than cationic and nonionic ones for Na bentonite suspension. The dual-flocculation of cationic and anionic flocculant combinations and pre-destabilization via coagulants of Na bentonite suspension were also studied. Better flocculation performance was achieved with these combined methods.
KEYWORDS Bentonite; coagulation- flocculation; conductivity; mono-multivalent cations; zeta potential 1. Introduction
Dewatering of concentrates and tailings is an important process in most mineral processing plants. Coagulation and/ or flocculation are the most common processes to remove very fine particles from water and wastewater. During dewatering, inorganic coagulants are commonly used due to their low cost and ease of use. However, their application is constrained due to low flocculating efficiency. Polymeric flocculants have become very popular in industrial effluent treatment because of their high efficiency at low dosage (Lee, Robinson, and Chong 2014). However, they also have high cost, non- biodegradability, and produce large amounts of sludge that is not easily dewatered and is unfriendly to the environment. Bentonite is common in mining tailings (Mierczynska- Vasilev et al. 2013). The major component of bentonite is montmorillonite, which is a smectite clay mineral that consists of one octahedral layer between two silicon-oxide tetrahedral layers. Smectites have very small particle sizes (0.01–1 µm) with a high specific surface area (700–800 m2/g); their cation exchange capacities are between 80 and 100 meq/100 g (Duman and Tunç 2009; Zadaka, Radian, and Mishael 2010). Bentonites are classified as highly viscous - swelling Na bentonite and mixed type bentonite (Na-Ca bentonite), and low viscous -less swelling Ca bentonite.
The swelling behavior of minerals affects the rheology of the suspension by increasing its viscosity. In this case, the coagulation/flocculation behavior of the suspension may com-pletely change, and thus the swelling character of the bentonite becomes very effective during the coagulation and flocculation
processes. In industrial applications of a solid-liquid separation, it is well known that the clay particles—especially those with ultra-fine sizes—are resistant to flocculation. Their presence can cause a highly problematic dewatering process, high flocculant demand, low settling rates, and poor supernatant clarity. Therefore, the polymer consumption in the flocculation of montmorillonite-rich suspensions is much higher than the economical limit (Addai-Mensah 2007; Cirak
2010). Recent studies have shown that coagulation plus floccu-lation (Ahmad et al. 2008; Sher, Malik, and Liu 2013; Lee, Robinson, and Chong 2014) and dual flocculation (Yoon and Deng 2004; Sanga and Xiao 2008; Lemanowicz et al.
2011) results in better sedimentation efficiency rather than single methods.
In this study, the performance of single and combined coagulation and flocculation methods on the sedimentation efficiency of bentonite samples exhibiting different swelling potentials (Na and Ca bentonite) was investigated. The zeta potential, ζ, is an important electrokinetic property of clay minerals, and it plays a significant role in understanding the adsorption mechanism of inorganic and organic molecules within the solid/solution interface. The sign and magnitude of ζ can be used to estimated the colloidal dispersion stability and the particle affinity for certain substances (Ersoy and Celik
2002; Duman and Tunç 2009; Zadaka, Radian, and Mishael
2010; Vercellone, Sham, and Torres 2015). The effects of coagulants and flocculants on the zeta potential of Na and Ca bentonite were determined, and the data revealed that the electrokinetic properties of fine particles in an aqueous
none defined
CONTACT V. Onen vonen@selcuk.edu.tr Department of Mining Engineering, Selcuk University, Konya 42075, Turkey.
© 2018 Taylor & Francis
solution play a significant role in coagulation and flocculation mechanisms. We also studied the role of the change in conductivity on the efficiency of the process.
2. Materials and methods
2.1. Materials
The coagulation and/or flocculation experiments were conducted using bentonite samples exhibiting different swelling potential (Na and Ca bentonite). The Na bentonite samples with a high swelling capacity were obtained from Karakaya Bentonite Inc. Co. The low swelling capacity Ca bentonite samples were taken from Canbensan Inc. Co. The montmorillonite contents of Na and Ca bentonites were ˃90% and 76–80%, respectively. The general properties of the minerals are shown in Table 1. Particle size analysis of bentonite powder used a Sympatec Helos H1735 particle size analyzer, and 75% of particles were less than 50 µm.
The Al2(SO4)3, FeCl3, MgCl2, CaCl2, and NaCl were used as coagulants in the coagulation tests. Polyacrylamide-based floc-culants were used in the flocculation tests. Anionic (A-150), cationic (C-521), and nonionic (N-100) Superfloc flocculants were obtained from American Cyanamid Company (Cytec). The molecular weights of A-150 and N-100 polymers are 5-15 �106 and C-521 is 10 � 103 − 0.5 � 106. In addition, the catio-nic polymer has a very high charge (100%), and the aniocatio-nic charge density of A-150 polymer is 50 to 60%. The properties of the polymeric flocculants have been reported by the manufacturer (Ozkan et al. 2016).
The coagulants and flocculants used in the experiments were prepared as 5 - 0.01% solutions. Stock solutions of the coagu-lants and floccucoagu-lants were prepared with distilled water and diluted to the required concentrations. Sodium hydroxide or hydrochloric acid (Merck) solutions were used for pH adjust-ments, and pH was measured using a Jenco-6230 pH meter. The turbidity of the supernatant was measured with a Velp Scientifica turbidimeter. The zeta potentials of the samples were determined using a Zeta Plus apparatus from Brookhaven.
2.2. Methods
The experiments were performed in 600 mL beakers on a mechanical stirrer. First, Ca and Na bentonite suspensions
were prepared at a 2.5% solid ratio. The pH of the prepared Ca and Na bentonite suspensions were 8.61 and 9.47, respect-ively. The mixture of the clay/water suspension was pre- conditioned for 15 min at 850 rpm to obtain a well-dispersed suspension. The turbidity of well-dispersed Ca and Na suspensions were 1372 and 6190 NTU, respectively. Before performing the coagulation/flocculation experiments, dispersed suspensions were allowed to settle naturally for 30 min. At the end of the 30 min of natural settling, the sus-pension turbidity of the Ca and Na bentonites were 635 and 5400 NTU, respectively. These values were used as initial turbidities.
In coagulation and/or flocculation experiments, the suspensions were first pre-conditioned for 15 min at 850 rpm. Next, the speed was reduced to 200 rpm, and the coagulant/flocculant was added. The suspension was stirred for 3 min and allowed to settle. After 30 min, 10 ml of super-natant samples were removed from a fixed distance below the air-liquid interface, and the turbidity, zeta potential, and con-ductivity analyses were conducted. In the dual-flocculation experiments, an additional conditioning period of 3 min was used for each coagulant or flocculant.
The performance of the sedimentation processes was evaluated using Eq. (1) (Osborne 1978; Ozkan and Yekeler
2004);
Sedimentation; % ¼ ½ T0 Tf
�
=T0� �100 ð1Þ
where T0 is the initial turbidity (nephelometric turbidity unit, NTU) of the suspension (at the end of the 30 min settling time), and Tf is the turbidity of the supernatant after the process. The accuracy was �4%.
3. Result and discussion
3.1. Zeta potential measurements
The zeta potentials of the clay samples as a function of pH are given in Figure 1. The electrical charge of montmorillonite originates from (pH-dependent) proton adsorption/desorp-tion reacadsorption/desorp-tions on the surface’s hydroxyl groups and isomorphous substitutions within the clay structure. Although the structural negative charges are not in direct contact with the aqueous solution, they do induce a net negative electrical
Table 1. General properties of bentonite samples.
Mineral Ca Bentonite Na Bentonite Humidity (%) 12.15 8 Swelling index (ml) 7 22 Viscosity 600 rpm 4 46 Filtration volume (ml) 48 11.2 Plastic viscosity 2 15.6 Yield point 0 0.4 Chemical Analysis(%) SiO2 71.50 61.28 Al2O3 15.20 17.79 Fe2O3 1.28 3.01 CaO 1.87 4.54 Na2O 0.41 2.70 MgO 2.48 2.10 K2O 1.15 1.24 TiO2 0.15 – P2O5 0.01 –
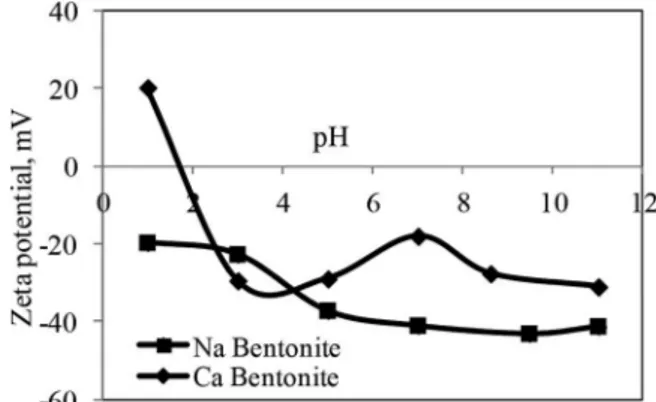
Figure 1. Zeta potential of the Ca and Na bentonite samples as a function of pH.
potential that is primarily responsible for the electrical, sorptive, and coagulative properties of the particles. Conse-quently, the montmorillonite ζ remains practically constant (and negative) in a broad range of pH values. This is typical for particles with a structural negative charge. (Avena and De Pauli 1998; Duc, Gaboriaud, and Thomas 2005; Vercellone, Sham, and Torres 2015).
The IEP value of Na bentonite could not be determined because it shows a negative electrical charge at all pH values. The zeta potential of the particles increased in negative values along with an increase in the suspension pH. The IEP value of Ca bentonite was 1.9. At the natural pH of Ca and Na benton-ite suspensions (pH 8.61–9.47), the zeta potential values were determined to be approximately −27 mV and −43 mV, respectively. The clay suspensions had a relatively high stab-ility due to the high negative surface charge of the particles.
3.2. Natural sedimentation of bentonite suspensions without treatment
Ca and Na bentonite suspensions whose initial turbidity were 1372 and 6190 NTU were allowed to settle naturally under quiescent conditions for 180 min, and the change of supernatant turbidity with time was followed (Figure 2). The supernatant turbidity value decreased slowly with increasing settling time and reached 189-4210 NTU at a settling time of 180 min. During this period, the turbidity removal
efficiency for Ca and Na bentonite was 86% and 32%, respect-ively. This indicated that natural settling was insufficient, and treatment was required.
3.3. Effects of coagulant/flocculant concentration on turbidity and conductivity of bentonite suspensions
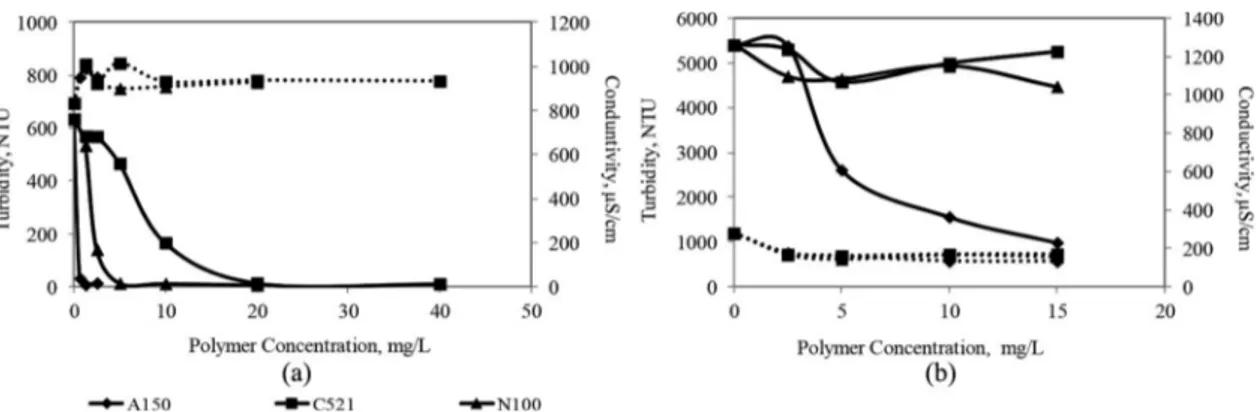
Figure 3 shows the effects of the coagulant concentrations on conductivity and turbidity of bentonite suspensions at natural pH. The turbidity of the Ca bentonite suspension decreased rapidly with increasing coagulant concentration. In the coagu-lation of a Ca bentonite suspension, the best performance (98.5%- 2.87 NTU) was obtained with Al2(SO4)3 at 1000 mg/ L. All coagulants have 85% efficiency and above. The effective-ness of the coagulants increased with increasing ionic value of metal salts (Figure 3a). However, the same coagulants did not provide efficiency for Na bentonite suspension. In the Na bentonite suspension, the best performance (22% efficiency-4220 NTU) was obtained at 750 mg/L FeCl3 (Figure 3b). Abu-Jdayi (2011) showed that the bentonite-water suspension exhibits a non-Newtonian behavior and the degree of thix-otropy increases with increasing Na/Ca ratio in bentonite. Consequently, thixotropy of Na bentonite is greater than Ca bentonite. Therefore, the high swelling property of the Na montmorillonite causes a high yield stress, and this situation challenges the sedimentation process of the Na bentonite sus-pensions. In addition, the surface area of Na bentonite is larger than Ca bentonite; therefore, the Na bentonite may need more coagulants/flocculants. In addition, the coagulants destabilized the Na bentonite suspension at higher concentrations, however it is rare to use such high dosages industrially.
The turbidity of the Ca bentonite suspension decreased rapidly with the increasing concentration of flocculants. All flocculants achieved approximately 98% flocculation efficiency (Figure 4a). On the other hand, a significant performance was not obtained with C-521 and N-100 in Na bentonite suspen-sion. The highest flocculation efficiency (81%) was achieved with A-150 at 15 mg/l which is followed by N-100 (17%) and C-521(15%) (Figure 4b). The cationic flocculant appeared to be a suitable choice when the particles are negatively charged due to electrostatic interaction. However, flocculant adsorption is not only related to functional interactions with the surface, but also to the molecular weight of the flocculant
Figure 2. Change of suspension turbidities with time under natural settling conditions.
Figure 3. Effects of coagulant concentration on the turbidity and conductivity of (a) Ca bentonite and (b) Na bentonite suspensions; _____ Turbidity; ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ Conductivity.
and the rate of polymer chain dissolution. High molecular weight flocculants are extremely effective at promoting floc growth in suspensions (Hogg, Bunnaul, and Suharyono
1993; Owen et al. 2002). They can be adsorbed onto several particle surfaces simultaneously and become a three- dimensional matrix. The C-521 flocculant has a lower molecular weight versus A-150; therefore, it was not effective in flocculating the Na bentonite suspension. Here, the polymer bridging mechanism is of primary importance, whereas charge neutralization will be of secondary or only minor importance. Several studies have shown that high molecular weight anionic polymers (e.g., polyacrylamide) are commonly used to settle negatively charged clays (Hogg 2000; Patience, Addai-Mensah, and Ralston 2003; Nasser and James 2006). High molecular weight anionic polyacrylamides increase the settling rate and eliminate the restabilization of particles by excessive polymer adsorption; therefore; they are more effective than cationic polymers. This is due to the limited polymer adsorption allowed by the repulsion between the anionic polyacrylamide and the particles. However, large open structure flocs are pro-duced from loops and tails that form as a result of polymer molecule expansion due to charge repulsion. This phenom-enon is influential during the flocculation of negatively charged clay dispersions (Nasser and James 2006).
The conductivity of the Ca and Na bentonite suspensions were 834 and 279 µS/cm, respectively, at their natural states. This showed that the ions in the Na bentonite had a limited passage to suspension with low solubility. While the Ca ben-tonite particles were suspend in an aggregated form of several unit layers due to the bivalent calcium ions, the Na bentonite particles do this in the form of a unit layer (Kim and Palomino
2009). In addition, the conductivity difference between these minerals is related to a higher swelling and water holding capacity of the Na bentonite. Conductivity falls as the water content in the structure rises. The electrical conductivity of clays depends on the volume conductivity (∼water content and composition) and on surface conductivity (∼diffuse double layer, DDL; e.g.,) (Tabbagh and Cosenza 2007; Kaufhold et al. 2015). The conductivity values decrease for all coagulants—especially in lower concentrations because the Na bentonite did not effectively precipitate, and the particles were suspended. The conductivity increased as the amount of coagulants included in the suspension increased. Salts are ionic compounds, and form (+) and (-) charges when
dissolved in water; hence, they contribute to electrical conductivity. During coagulation, anions remain in the solution while cations are adsorbed onto the particle surface. As a result, conductivity increases with increasing coagulant concentration. Electrical conductivity indicating the dissolved mineral content of the water; in other words, it reflects the ionic strength. Water conductivity depends on total and rela-tive concentration as well as mobility and valency of the ions in the water. NaCl is the coagulant with the highest increased conductivity of suspension (Figure 3a,b). NaCl is an alkali metal salt, and alkali metal salts (except lithium) are 100% separated into their ions. Thus, they produce more ions than other metal salts, and they increase the conductivity of the medium the most. In addition, other coagulants are found at a lower level in the ionic form within the suspension because they form a precipitate during coagulation. As a result, NaCl increases the electrical conductivity. The flocculant concen-tration did not affect suspension conductivity (Figure 4a,b).
3.4. Effects of coagulant/flocculant concentration on zeta potential of bentonite suspensions
Zeta potential measurements were used to estimate the interactions between Na/Ca–bentonite and coagulant/ flocculant as a function of coagulant/flocculant species and concentration. The clay minerals consist of cations (Si, Al+ Mg+2 and others) and anions (O and OH), and there are bro-ken bonds on their corners, edges, and surfaces. It assumed that the bonds on the broken edges of the clay minerals are especially positively charged at acidic and neutral pH values. In other words, it presumed that the clay surfaces generally carry negative charges, whereas their edges consist of posi-tively charged cations (Akin and Celik 1995; Evcin and Kavas
2004). This means that the value obtained from the zeta poten-tial measurements are an average. The Smoluchovski equation is used to calculate the electrophoretic mobility and zeta potential for spherical particles. However, zeta potential mea-surements can be highly misleading when used to estimate the surface charge distribution of anisotropic minerals (Missana and Adell 2000; Burdukova, Bradshaw, and Laskowski 2007; Hou et al. 2009; Forbes, Davey, and Smith 2014). Nevertheless, the zeta potential is commonly used to estimate the behavior of clay mineral that may interact with other minerals depending on their charges.
Figure 4. Effects of flocculant concentration on the turbidity and conductivity of (a) Ca bentonite and (b) Na bentonite suspensions; __________ Turbidity; ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐‐ Conductivity.
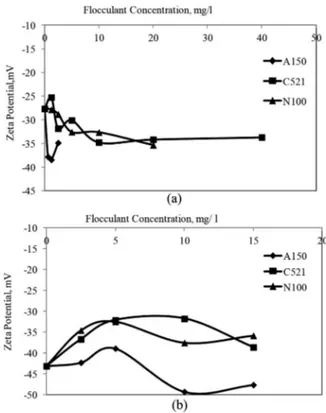
The electrical layer-suppressing effect of the cations for Ca bentonite started to become effective at a certain cation con-centration (Figure 5a). The zeta potential remained constant or reduced slightly after an increase of a certain concentration for Na bentonite except for NaCl (Figure 5b). Previous studies have shown that as compared to monovalent cations, divalent and trivalent cations can make the clay zeta potential closer to zero as predicted by DLVO theory describing the coagulation of bentonite suspension dispersions (Lagaly and Ziesmer 2003; Duman and Tunç 2009; Zadaka, Radian, and Mishael 2010). Charged particles are stabilized by electrostatic repulsion between the diffuse electrical double layers surrounding the particles. The double layer compression is the main mech-anism for decreasing the stability of the colloidal particles (García-García, Jonsson, and Wold 2006). The inorganic ions in the suspension compress the electrical double layer—this minimizes the zeta potential and interparticle repulsion. The zeta potential approached to zero with an increasing electro-lyte concentration, but this change was faster with increasing valency of the counter-ions. If the particles have zeta potential close to zero then there will be no force to prevent the particles from coming together. The dividing line between stable and unstable suspensions is generally considered to be either +30 or 30 mV (Duman and Tunç 2009). No coagulation was seen because the zeta potential values for Na bentonite suspensions were not in this range (Figure 5b).
In the flocculation of the Ca bentonite suspension, the zeta potential decreased further with increasing concentration for all flocculants (Figure 6a). The same situation was observed with the use of anionic flocculant with Na bentonite (Figure 6b). In the case of anionic flocculants, the small decrease in the zeta potential with increasing flocculant con-centration did not arise from charge neutralization, but rather came from the shift in position of the shear plane due to the
adsorbed layer. This is due to the negative polymer chains (Patience, Addai-Mensah, and Ralston 2003; Nasser and James
2006). Flocculation of fine particles occurs by polymer bridg-ing, charge neutralization, polymer particle surface complex formation, depletion flocculation, or by a combination of these mechanisms (Gregory 1985; Mpofu, Addai-Mensah, and Ralston 2003; 2004). Zeta potential measurements indicated that a polymer-bridging mechanism played a more significant role in the flocculation of Ca and Na bentonite suspensions.
3.5. Sedimentation of Na bentonite with combine methods
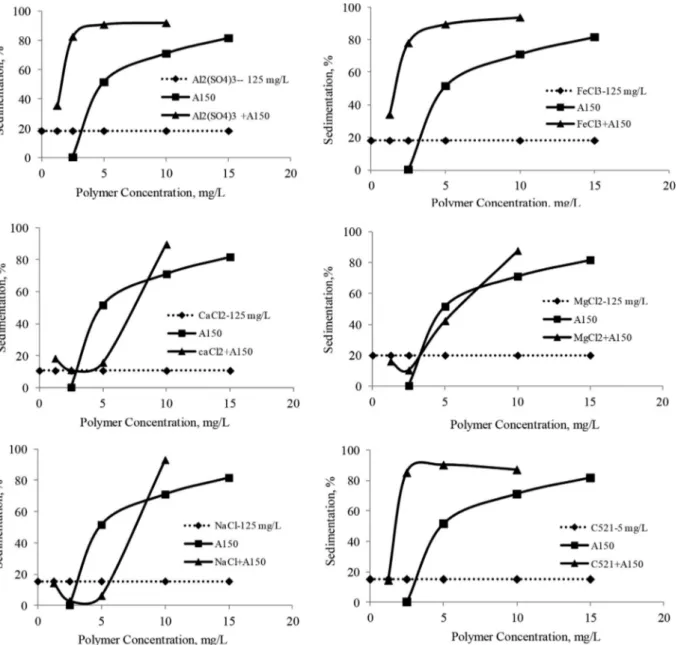
No coagulation were observed within the range of coagulant concentrations studied here for Na bentonite suspensions. Therefore, coagulants seemed to have no effect on the sedimentation of Na bentonite. In the flocculation of the Na bentonite suspension, the most effective anionic flocculant provided 81% efficiency. Flocculation efficiency can be rapidly increased with increasing A-150 flocculant concentration. However, the flocculant consumption may exceed the limits in this case. Therefore, coagulation plus flocculation and dual flocculation (cationic plus anionic flocculant) experiments were conducted at the optimum concentrations determined in previous coagulation and flocculation experiments.
These data clearly showed that coagulation plus flocculation and dual flocculation methods improved the sedimentation of Na bentonite suspension versus a single methods. At lower concentrations, the efficiency of trivalent cations and cationic flocculants was particularly increased. Thus, the amount of flocculant required was reduced. Furthermore, the increase
Figure 5. Effect of coagulant concentration on the zeta potential of (a) Ca bentonite and (b) Na bentonite suspensions.
Figure 6. Effect of flocculant concentration on the zeta potential of (a) Ca bentonite and (b) Na bentonite suspensions.
in the A-150 concentration strongly improved the flocculation of the Na bentonite suspension (Figure 7). These inorganic cations reduced the negative surface charge of the particles and supported the adsorption of the A-150 polymer onto the particle surface thanks to the combined effects of charge neutralization and bridging mechanism. The cationic floccu-lant produced small, tight flocs, and the anionic floccufloccu-lant produced large, loose flocs. Therefore, we can state that that the combination of cationic and anionic flocculants can pro-duce large and tight flocs. As a result, the anionic polymer
provided better interparticle bridging when adsorbed on the particles with a pre-adsorbed polymer of cationic charge. (Alam et al. 2011; Ozkan et al. 2016). An additional increase of approximately 10% was seen at lower concentrations by applying combined methods. The flocculation performance was characterized by measuring the sedimentation rate as well as the residual turbidities (Gregory and Barany 2011). In the applications with a combined method, the Na bentonite suspension’s rate of sedimentation also increased for all chemicals (Table 2).
Figure 7. Effect of anionic flocculant concentration on sedimentation of Na bentonite suspension in coagulation plus flocculation and dual flocculation methods.
Table 2. Comparison of single methods with combined methods.
A150 Al2(SO4)3 FeCl3 CaCl2 MgCl2 NaCl C521
F C C + F C C + F C C + F C C + F C C + F Mono Dual Opt con. (mg/L) 15 125 5 750 5 125 10 125 10 375 10 5 5 Turbidity (NTU) 988 4410 496 4220 570 4820 565 4320 665 4300 373 4580 553 Efficiency (%) 81.70 18.3 90.8 21.85 89.4 10.74 89.5 20 87.7 20.37 93.1 15.19 90.13 Settling velocity (mm/min) 0.47 0.16 1.31 0.16 1.36 0.10 1 0.10 0.31 0.16 0.94 0.10 0.84 F, Flocculation; C, Coagulation; C + F, Coagulation + Flocculation.
4. Conclusion
All coagulants and flocculants studied here more strongly -coagulated and flocculated the colloids in the Ca bentonite suspension than colloids in the Na bentonite suspension. The highest coagulation efficiencies were obtained with Al2 (SO4)3 (98.5%) and FeCl3 (22%) for Ca and Na bentonite sus-pensions, respectively. All flocculants provided about 98% efficiency for Ca bentonite suspension; the highest efficiency (81%) was achieved with an anionic flocculant for Na benton-ite suspension. Cationic (15%) and nonionic (17%) flocculants were not effective in the flocculation of Na bentonite suspen-sion colloids. The swelling character of the Na bentonite causes a high yield stress, and this challenges the sedimen-tation process.
The conductivity of the coagulation/flocculation effluents is a very important parameter especially when this effluent is recycled to the plant. The suspension conductivity increased with increasing coagulant concentration. The NaCl is the coagulant that provides the highest conductivity to the suspension. Flocculants have no significant effect on suspen-sion conductivity. The activity of coagulants is increased by the increased ionic value of metal salts. In coagulation, multi-valent cations have a greater influence on the zeta potential of the samples than monovalent cations. Zeta potential measure-ments indicated that a polymer bridging mechanism played a more significant role in flocculation.
The higher flocculation performances could be achieved with combined methods at lower coagulant and flocculant concentrations compared to single coagulation or flocculation methods. In the combined coagulation/flocculation applica-tions, the elimination of the charge barrier between the clay particles in the suspension by the addition of a cationic poly-mer or an inorganic cation supports the bridging action and adsorption of the anionic polymer. Consequently, both the 10% increase in efficiency and the increase in the sedimen-tation velocity were obtained with combined methods. The ideal situation is sedimentation of the highest amount of clay minerals with the highest possible flocculation efficiency by lowest polymer concentration. Therefore, the determination of the optimum flocculation conditions is crucial.
Funding
This study was financially supported by the Coordinatorship of Selcuk University’s Scientific Research Projects under grant no 15201055.
References
Abu-Jdayi, B. 2011. Rheology of sodium and calcium bentonite–water dispersions: Effect of electrolytes and aging time. International Journal
of Mineral Processing 98:208–13. doi:10.1016/j.minpro.2011.01.001. Addai-Mensah, J. 2007. Enhanced flocculation and dewatering of clay
mineral dispersions. Powder Technology 179:73–78. doi:10.1016/j.
powtec.2006.11.008.
Ahmad, A. L., S. S. Wong, T. T. Teng, and A. Zuhairi. 2008. Improvement of alum and PACl coagulation by polyacrylamides (PAMs) for the treatment of pulp and paper mill. Chemical Engineering Journal 137:510–7. doi:10.1016/j.cej.2007.03.008.
Akin, Y., and M. S. Celik. 1995. Electrokinetic behaviours of montmoril-loite type clays. Proceedings of the Ind. Min. Symp., ed. by Köse and Kızıl, İzmir-Turkey, April 21–22.
Alam, N., O. Ozdemir, M. A Hampton, and V. Nguyen. 2011. Dewatering of coal plant tailings: Flocculation followed by filtration. Fuel 90 (1): 26–35. doi:10.1016/j.fuel.2010.08.006.
Avena, M. J., and C. P. De Pauli. 1998. Proton adsorption and electrokinetics of an argentinean montmorillonite. Journal of Colloid
and Interface Science 202:195–204. 0021–9797/98.
Burdukova, E., D. J. Bradshaw, and J. S. Laskowski. 2007. Effect of CMC and pH on the rheology of suspensions of isotropic and anisotropic minerals. Canadian Metallurgical Quarterly 46:273–8. doi:10.1179/
cmq.2007.46.3.273.
Cirak, M. 2010. Flocculation behaviour of two different clay samples from kırka tincal deposit. Msc thesis, The Graduate School of Applied and Natural Sciences of Middle East Technical University, 132.
Duc, M., F. Gaboriaud, and F. Thomas. 2005. Sensitivity of the acid–base properties of clays to the methods of preparation and measurement:1. Literature review. Journal of Colloid and Interface Science 289:139–47. doi:10.1016/j.jcis.2005.03.060.
Duman, O., and S. Tunç. 2009. Electrokinetic and rheological properties of Na-bentonite in some electrolyte solutions. Microporous and
Mesoporous Materials 117:331–38. doi:10.106/j.micromeso.2008. 07.007.
Ersoy, B., and M. S. Celik. 2002. Electrokinetic properties of clinoptilolite with mono-and multivalent electrolytes. Microporous and Mesoporous
Materials 55:305–12. PII: S1387–1811(02)00433-x.
Evcin, A., and T. Kavas. 2004. Investigation of the effect of different deflocculants on viscosity of slurry prepared with Söğüt and Konya clays. Proc of the 5th Ind. Min. Symp., İzmir-Turkey, May 13–14.
Forbes, E., K. J. Davey, and L. Smith. 2014. Decoupling rehology and slime coatings effect on the natural flotability of chalcopyrite in a clay-rich flotation pulp. Minerals Engineering 56:136–44. doi:10.1016/
j.mineng.2013.11.012.
García-García, S., M. Jonsson, and S. Wold. 2006. Temperature effect on the stability of bentonite colloids in water. Journal of Colloid and
Interface Science 298:694–705. doi:10.1016/j.jcis.2006.01.018. Gregory, J. 1985. The use of polymeric flocculants. Proc. of the
Engineering Foundation Conference on Flocculation, Sedimentation and Consolidation, The Clister Sea Island, Georgia, USA. American Institute of Chemical Engineers, New York, USA, 125–37.
Gregory, J., and S. Barany. 2011. Adsorption and flocculation by polymers and polymer mixtures. Advances in Colloid and Interface Science 169:1–12. doi:10.1016/j.cis.2011.06.004.
Hogg, R. 2000. Flocculation and dewatering. International Journal of
Mineral Processing 58:223–36. PII: S0301–7516(99)00023-x.
Hogg, R., P. Bunnaul, and H. Suharyono. 1993. Chemical and physical variables in polymer-induced flocculation. Minerals and Metallurgical
Processing 10:81–85.
Hou, J., H. Li, H. Zhu, and L. Wu. 2009. Determination of clay surface potential: A more reliable approach. Soil science society of America
Journal- Soil Chemistry 73 (5):1658–63. doi:10.2136/sssaj.2008.0017. Kaufhold, S., R. Dohrmann, M. Klinkenberg, and U. Noell. 2015.
Electrical conductivity of bentonites. Applied Clay Science 114: 375–85. doi:10.1016/j.clay.2015.05.032.
Kim S., and A. M. Palomino. 2009. Polyacrylamide-treated kaolin: A fabric study. Applied Clay Science 45 (4):270–9. doi:10.1016/j.clay.2009. 06.009.
Lagaly, G., and S. Ziesmer. 2003. Colloid chemistry of clay minerals: The coagulation of montmorillonite dispersions. Advances in Colloid and
Interface Science 100–102:105–28. PII: S0001–8686(02)00064–7.
Lee, C. S., J. Robinson, and M. F. Chong. 2014. A review on application of flocculants in wastewater treatment. Process Safety and Environmental
protection 92:489–508. doi:10.1016/j.psep.2014.04.010.
Lemanowicz, M., Z. Jach, E.Kilian, and A. Gierczycki. 2011. Ultra-fine coal flocculation using dual-polymer systems of ultrasonically conditioned and unmodified flocculant. Chemical Engineering Journal 168:159–69. doi:10.1016/j.cej.2010.12.057.
Mierczynska-Vasilev, A., M. Kor, J. Addai-Mensah, and D. A. Beattie. 2013. The influence of polymer chemistry on adsorption and flocculation of talc suspensions. Chemical Engineering Journal 220:375–382. doi:10.1016/j.cej.2012.12.080.
Missana, T., and A. Adell. 2000. On the applicability of DLVO theory to the prediction of clay colloids stability. Journal of Colloid and Interface
Science 230 (1):150–6. doi:10.1016/jcis.2000.7003.
Mpofu, P., J. Addai-Mensah, and J. Ralston. 2003. Investigation of the effect of the polymer structure type on flocculation rheology and dewatering behaviour of kaolinite dispersions. International Journal
of Mineral Processing 71:247–68. doi:10.1016/S0301-7516(03)00062-0. Mpofu, P., J. Addai-Mensah, and J. Ralston. 2004. Flocculation and dewa-tering behaviour of smectite dispersions: Effect of polymer structure type. Minerals Engineering 17:411–23. doi:10.1016/j.mineng.2003. 11.010.
Nasser, M. S., and A. E. James. 2006. The effect of polyacrylamide charge density and molecular weight on the flocculation and sedimentation behaviour of kaolinite suspensions. Separation and purification
Technolog, 52:241–52. doi:10.1016/j.seppur.2006.04.005.
Osborne, D. G. 1978. Recovery of slimes by a combination of selective flocculation and flotation. Transations of the Institution of Mining
and Metallurgy. Section C 87:189–93.
Owen, A. T., P. D. Fowel, J. D. Swift, and J. B. Farrow. 2002. The impact of polyacrylamide flocculant solution age on flocculation performance.
International Journal of Mineral Processing 67:123–44. PII: S0301–7516
(02)00035–2.
Ozkan A., and M. Yekeler. 2004. Coagulation and flocculation character-istics of celestite with different inorganic salts and polymers. Chemical
Engineering and Processing 43:873–9. doi:10.1016/S0255-2701(03) 00107-7.
Ozkan, A., B. Oner, V. Onen, and S. Duzyol. 2016. Flocculation of coal suspension with mono/dual polymer systems and contribution of Ca
(II)/Mg (II) ions. Seperation Science and Technology 51:106–14.
doi:10.1080/01496395.2015.1085876.
Patience, M., J. Addai-Mensah, and J. Ralston. 2003. Investigation of the effect of polymer type on flocculation, rheology and dewatering behaviour of kaolinite dispersions. International Journal of Mineral
Processing 71:247–68. doi:10.1016/S0301-7516(03)00062-0.
Sanga, Y., and H. Xiao. 2008. Clay flocculation improved by cationic poly(vinyl alcohol)/anionic polymer dual-component system. Journal
of Colloid and Interface Science 326:420–5. doi:10.1016/j.jcis.2008. 06.058.
Sher, F., A. Malik, and H. Liu. 2013. Industrial polymer effluent treatment by chemical coagulation and flocculation. Journal of Environmental
Chemical Engineering 1:684–9. doi:10.1016/j.jece.2013.07.003. Tabbagh, A., and P. Cosenza. 2007. Effect of microstructure on the
electrical conductivity of clay-rich systems. Physics and Chemistry of
the Earth 32:154–60. doi:10.1016/j.pce.2006.02.045.
Vercellone, S. Z., E. Sham, and E. M. F. Torres. 2015. Measure of zeta potential of titanium pillared clays. Procedia Materials Science 8: 599–607. doi:10.1016/j.mspro.2015.04.114.
Yoon, S. Y., and Y. Deng. 2004. Flocculation and reflocculation of clay suspension by different polymer systems under turbulent conditions.
Journal of Colloid and Interface Science 278:139–45. doi:10.1016/j. jcis.2004.05.011.
Zadaka, D., A. Radian, and Y. G. Mishael. 2010. Applying zeta potential measurements to characterize the adsorption on montmorillonite of organic cations as monomers, micelles, or poly-mers. Journal of Colloid and Interface Science 352:171–7. doi:10.1016/j.jcis.2010.08.010.