Contents lists available atScienceDirect
Behavioural Brain Research
journal homepage:www.elsevier.com/locate/bbrResearch report
Voluntary, involuntary and forced exercises almost equally reverse
behavioral impairment by regulating hippocampal neurotrophic factors and
oxidative stress in experimental Alzheimer’s disease model
Muaz Belviranlı
⁎, Nilsel Okudan
Selçuk University, School of Medicine, Department of Physiology, Konya, Turkey
A R T I C L E I N F O Keywords: Alzheimer's disease Voluntary exercise Involuntary exercise Forced exercise Oxidative stress Neurotrophic factors A B S T R A C T
The purpose of this study was to compare the neuroprotective effects of voluntary, involuntary, and forced exercise trainings on behavioral impairment as well as hippocampal Alzheimer’s disease (AD) pathology and oxidative stress markers, and levels of neurotrophic factors in the rat model of AD. The rats were assigned to control, Alzheimer model, Alzheimer + voluntary exercise, Alzheimer + involuntary exercise, or Alzheimer + forced exercise group. The rat model of AD was established by D-(+)-Galactose (D-GAL) and AlCl3
administration for 90 days. Voluntary, involuntary (swimming) or forced exercise (load-swimming) trainings were performed for 90 days starting with the D-GAL and AlCl3administration and then several behavioral tests
were applied. Locomotor activity, exploratory behavior, and spatial memory were lower but anxiety levels were higher in the Alzheimer model group, than in the other groups (P < 0.05). The hippocampal levels of the amyloid beta 1–42, microtubule associated protein Tau, malondialdehyde, and protein carbonyl levels were higher, but brain-derived neurotrophic factor, nerve growth factor, glutathione and superoxide dismutase levels were lower in the Alzheimer model group, than in the other groups (P < 0.05). The results of the present study suggest that all exercise modalities almost equally attenuated non-cognitive and cognitive disturbances in a rat model of AD. Elevated neurotrophic factors, and improved oxidative stress could mediate these beneficial effects.
1. Introduction
Alzheimer’s disease (AD) is a multifactorial, age-related progressive neurodegenerative disorder, and is the most prevalent form of dementia [1]. There are many theories for the reason for AD, such as amyloid, tau, oxidative stress, inflammatory, and epigenetics [2], of which the amyloid, tau, and oxidative stress theories have become the most widely accepted. According to these theories pathological accumulation of extracellular amyloid beta peptide (Aβ) and intracellular tau-con-taining neurofibrillary tangles [3], and imbalance between the pro-duction of reactive oxygen species (ROS) and the capacity of anti-oxidant defenses is responsible for the development of AD [4,5].
AD pathology influences different brain areas particularly hippo-campus, an important region responsible for the learning and memory, and one of the earliest brain structures that develop neurodegenerative changes in AD [6,7]. The pathogenesis of AD is closely associated with a series of neurodegenerative events in the hippocampus at cellular and
histomorphological level, such as microglial activation, neuroin-flammation, oxidative stress, metabolic energy failure, mitochondrial dysfunction, and consequent neuronal apoptosis [8–10]. Additionally, hippocampal cell proliferation, survival and neural differentiation have been observed to be reduced in rodent models of AD. Moreover, im-pairment in neural morphology and hippocampal neurogenesis have also been reported [6]. AD animals exhibit a marked impairment of cognitive functions such as learning and memory as shown in various behavioral tests including Morris water maze (MWM) [11]. In addition to cognitive functions, AD also negatively effects non-cognitive func-tions such as depression, anxiety, aggressiveness, and locomotion as shown in various behavioral tasks such as open field (OF), and elevated plus maze (EPM) [12].
Neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) are critical regulators of neu-rons and they play vital roles in neurogenesis, synapse formation, and learning and memory [13,14]. However, current evidences claim that a
https://doi.org/10.1016/j.bbr.2019.02.030
Received 12 October 2018; Received in revised form 15 February 2019; Accepted 17 February 2019
⁎Corresponding author at: Selçuk University, School of Medicine, Department of Physiology, Konya, 42131, Turkey.
E-mail address:mbelviranli@selcuk.edu.tr(M. Belviranlı).
Available online 18 February 2019
0166-4328/ © 2019 Elsevier B.V. All rights reserved.
reduction in the BDNF and NGF levels could be related with the pa-thogenesis of AD [15,16]. It has been demonstrated that hippocampal BDNF expression is reduced in the both experimental AD model [17] and in patients with AD [18]. Additionally, it has been showed that BDNF has particular and dose-dependent presentative impact against Aβ-induced neuronal toxicity [19], and suppression of the BDNF and NGF signaling elevates Aβ production [20].
Regular exercise is a non-pharmacological approach that prevents memory deterioration in the AD [6,21]. Many studies performed on rodents have demonstrated that regular exercise training improves neuroprotection [8], inflammation [8], and cognitive [9,11,22] and non-cognitive [12] functions via reducing Aβ and tau deposition. Ex-ercise training induced decrease in the Aβ and tau levels was related with the activation of the Aβ degradation enzymes [23,24], synaptic plasticity [25–27], neurogenesis [25,28], neurotrophic factors [27,29,30], and antioxidant systems [31,32], demonstrating that com-plex mechanisms may be responsible in the degradation of Aβ.
Various types of exercises such as swimming [33,34] treadmill running [11,35] and wheel running [34,36] has been studied in AD and all types of exercises improve behavioral impairment in AD. Wheel running is the only model used for voluntary exercise, whereas tread-mill running and swimming models generally are used for involuntary and forced exercises [34,37]. Compared with voluntary exercise, in-voluntary or forced exercises causes higher levels of stress and this si-tuation induces hippocampal corticotropin-releasing hormone (CRH) secretion [28,38,39]. Increased CRH concentration is thought to induce cognitive impairment [40] and therefore forced exercise might nega-tively affect cognitive and non-cognitive functions [41]. However, an-imals might become accustomed if involuntary or forced exercise-in-duced stress was repeated for a sufficient time, as postulated in the habituation theory [42]. Beneficial effects of exercise training were observed following just five weeks of treadmill running up to nine months of wheel running in rodents [6]. AD pathologies are reduced by exercise training when introduced both prior to disease onset or starting after the onset of disease in rodents and in humans. Therefore, exercise training can be used a preventative or therapeutic intervention [9].
In the present investigation, the rat model of AD was built up by consolidating D-(+)-galactose (D-GAL) with AlCl3, which could
di-minish the learning and memory capacities and caused to Aβ deposi-tion, increased APP expression, and other AD-like lesions [43]. To date various types of exercises such as swimming, treadmill running and wheel running have been used in the experimental AD model, and all exercise types improve cognitive and non-cognitive functions. How-ever, effectiveness of voluntary, involuntary and forced exercises has not been fully compared in the experimental AD model. This in-vestigation was designed to compare the effects voluntary, involuntary and forced exercise trainings on cognitive and non-cognitive functions. The MWM, OF and EPM tests were used to assess rats’ cognitive and non-cognitive performances. The hippocampal levels of the amyloid beta 1–42 (Aβ1–42) and microtubule associated protein Tau (MAPT), as a
marker of AD pathology, BDNF and NGF, as neurotrophic factors and malondialdehyde (MDA), protein carbonyl (PC), glutathione (GSH) and superoxide dismutase (SOD) as a marker of oxidative stress and anti-oxidant defense system was also evaluated. The comparison of the findings would give more information for the clinical practice in the future.
2. Methods 2.1. Animals
A total of 32 female Wistar rats (20 months old, weighing between 350–450 g at the beginning of the experiment) were taken from breeding colony of the Selçuk University Experimental Medicine Research and Application Center. The animals were housed in standard laboratory environments (temperature: 23 ± 1 °C, humidity: ˜50%, and 12:12 h light-dark cycle). The animals were given standard rat pellets and tap water ad libitum. All experimental procedures were approved by the Selçuk University Animal Care and Use Committee and followed the instructions from NIH Guideline for the Care and Use of Laboratory Animals.
Rats were assigned to the 5 groups as follows: Control (C, n = 6), Alzheimer model (ALZ, n = 6), Alzheimer + voluntary exercise model (ALZ + V-Ex, n = 6), Alzheimer + involuntary exercise model (ALZ + I-Ex, n = 8), and Alzheimer + forced exercise model (ALZ + F-Ex, n = 6). The percent lifespan of 20 months female Wistar rats is almost 75% in our lab. Since estrogen deprivation is one of the risk factors for the AD pathogenesis [44] in the present study aged female rats were used. Additionally, it has been reported that female rats enter menopause between the ages of 15 and 20 months [45].Fig. 1shows the time points of interventions and behavioral tests.
2.2. Alzheimer model establishment
The rat model of AD was established by intraperitoneal injection of D-GAL (60 mg kg−1; G0625, Sigma Chemical Co, St. Louis, MO, USA)
and intragastric administration of AlCl3 (5 mg kg−1; 294713, Sigma
Chemical Co, St. Louis, MO, USA) once a day for 90 days. Same volume of sterile physiological saline was given to the control group. The dose and duration were adopted according to the previous studies [46,47]. 2.3. Exercise protocols
The rats in the ALZ + V-Ex, ALZ + I-Ex, or ALZ + F-Ex group were performed exercise training for 90 days starting with the D-GAL and AlCl3administration. Rats in the ALZ + V-Ex group were housed in
pairs in cages with stainless-steel running wheels (MAY RW2508 Running Well, Commat, Ltd., Ankara, Turkey) and were allowed free access to the wheel 24 h per day. The ALZ + I-Ex group was subjected to swimming exercise 1 h d–1 for 90 days. Swimming exercise was
performed in a water-filled tank with a temperature maintained at 32 ± 2 °C. In the familiarization period, rats were acclimatized to the swimming training for 5 d (20 min d–1). The ALZ + F-Ex group forced
to swim against a load (5% of body weight) attached to the tail for 1 h, in the same tank conditions described above for involuntary exercise. 2.4. Behavioral tests
In order to examine and compare the effect of voluntary, in-voluntary and forced exercise trainings on non-cognitive and cognitive functions, a series of behavioral tests was performed starting with the 24 h after the last exercise session in the following order: OF, EPM and MWM.
Each experiment was video-recorded via computerized video
Fig. 1. Time points of interventions and behavioral tests.
OF: Open Field, EPM: Elevated Plus Maze, MWM: Morris Water Maze.
tracking system and analyzed with the Ethovision XT 9.0 system (Noldus Information Technology, Wageningen, The Netherlands). Behavioral tests were applied in a sound-and light-isolated condition. The OF and EPM apparatuses were cleaned with 70% ethyl alcohol and aired after each measurement. To reduce daily impact all tests were applied from the same experimenter at the same hours of the day. 2.4.1. Open field
The OF test is commonly used to assess locomotor activity, anxiety level and exploratory behavior in rodents. The OF equipment was made from the black square area (80 × 80 cm) surrounded by walls (40 cm high). Each rat was placed in the center of the box and allowed to ex-plore the environment freely for 5 min. Number of defecations, groomings, and rearings, time spent in the center (s), total distance traveled (cm), number of zone transition, time spent as a mobile (s), and velocity (cm s−1) were measured.
2.4.2. Elevated plus maze
To assess the anxiety level of the rats, an EPM test was performed. The EPM was a plus sign-shaped equipment that contains 2 open and 2 closed arms (50 cm × 10 cm length) and was placed 50 cm above the ground. The rat was placed on the intersection area of the four arms facing one of the open arms and was permitted to explore the maze for 5 min. During the test total distance traveled (cm), number of entries in closed arms, and open arms and mean velocity (cm s−1) were analyzed.
2.4.3. Morris water maze
The MWM is generally used to evaluate the spatial learning and memory function of the rats. The MWM experiment was performed in a water-filled (25 ± 1 °C) circular pool (150 cm diameter x 60 cm depth). During the MWM experiment two main tasks were performed in the following order:
2.4.3.1. Spatial navigation task. For these experiments, the pool was conceptually divided into four quadrants (northeast (NE), northwest (NW), southeast (SE) and southwest (SW)). A hidden square platform was placed approximately 2 cm below the surface of the water and it was located into the same quadrant (SE) for every task. In this task, the rats learned to find a hidden using extra-maze cues. The rats were performed 16 trials, which were averaged and presented as a daily block of four trails for 4 consecutive days. On each training day, four starting start positions were randomly used at once. For each trial, the animals were given 60 s to find the platform. If the rats were unsuccessful to find the platform within the allowed time period, it was physically placed on the platform by the experimenter for 30 s. During the test total distance (cm) traveled, time (s) to find the platform (latency), duration (s) of thigmotactic behavior (time the animals spent swimming close to the wall of the maze), and mean swimming speed (cm s−1) were recorded.
2.4.3.2. Probe task. At the 24 h after the spatial navigation trials a single 90 s probe trial task was performed in which platform was removed from the pool. Total distance traveled (cm), time spent in each quadrant (s), the number of platform crossing and (d) time spent in platform zone were recorded during the probe trial.
2.5. Biochemical examination 2.5.1. Hippocampal tissue sampling
The rats were sacrificed by decapitation under ether anesthesia 24 h after the last behavioral test. The brain tissues were quickly removed, and then the hippocampal tissues were immediately isolated form the brain, cleaned using ice-cold saline. Tissue samples were frozen in li-quid nitrogen and stored at −80 °C until biochemical analysis per-formed.
When assaying, 50 mg of frozen hippocampal tissue was added to
freshly prepared 20 volumes of ice-cold phosphate buffered saline (AMRESCO, VWR International, Solon, ON, USA, # E404-100TABS, NaCl 137 mM, KCl 2.7 mM, phosphate buffer 10 mM, pH: 7.4). Tissue samples were homogenized using the FastPrep®-24 system (MP
Biomedicals, Santa Ana, CA, USA). The homogenates were centrifuged at 12,000 g for 30 min at +4 °C, to obtain supernatants that were used for the analysis of the all biochemical variables.
2.5.2. Enzyme-linked immunosorbent assays (ELISA)
2.5.2.1. Measurement of AD pathology markers. For the analysis of the hippocampal levels of the AD pathology markers, the commercially available ELISA kits were purchased and used according to the manufacturers' instructions: The Aβ1–42 (Cat No: E-EL-R1402,
Elabscience Biotechnology, Co., Ltd, Wuhan, China), and MAPT(Cat
No: E-EL-R0943, Elabscience Biotechnology, Co., Ltd, Wuhan, China). The intra-assay CV of the kits was < 10%. Aβ1–42and MAPTlevels were
expressed as pg mg–1of protein.
2.5.2.2. Measurement of the neurotrophic factors. The BDNF and NGF concentrations in the hippocampus were measured using the commercially available rat BDNF (Cat No: E-EL-R1235, Elabscience Biotechnology, Co., Ltd, Wuhan, China) and NGF (Cat No: E-EL-R0652, Elabscience Biotechnology, Co., Ltd, Wuhan, China) ELISA kits, according to the manufacturer’s instructions. The intra-assay CV of the kits was < 10%. BDNF and NGF levels were expressed as pg mg–1of
protein.
2.5.2.3. Analysis of oxidative stress and antioxidant defense markers. Hippocampal lipid peroxidation level was evaluated by measuring the MDA concentration, using a Bioxytech MDA-586 assay kit (Cat. No. 21044, Oxis International Inc., Foster City, California, USA), according to the manufacturer’s instructions. MDA level was expressed as μmol MDA mg–1of protein.
PC level in the hippocampus was measured using a rat PC ELISA kit (Cat. No: 201-11-1700; Sunred Biological Technology, Shanghai, People’s Republic of China), according to the manufacturer’s instruc-tions. The intra-assay CV was < 10%. PC level was expressed as ng mg–1of protein.
Hippocampal GSH level was measured using a commercially avail-able kit (Cat. no. 703002, Cayman Chemical, Ann Arbor, MI, USA), according to the manufacturer’s recommendations. The intra-assay CV was 1.6%. GSH level was expressed as μmol mg–1of protein.
Total SOD activity (Cu/Zn, Mn, and FeSOD) in the hippocampal tissue was measured using a commercially available SOD assay kit (Cat. no. 706002, Cayman Chemical, Ann Arbor, MI, USA), according to the manufacturer’s recommendations. The intra-assay CV was 3.7%. SOD activity was expressed as U mg–1of protein.
2.5.2.4. Estimation of hippocampal protein content. The protein concentration of the supernatant was measured by the methods of Lowry et al. [48].
2.6. Statistics
The results were analyzed with SPSS v. 21.0 (IBM-SPSS Inc., Chicago, IL, USA). All data are presented as mean ± SEM. The nor-mality of the distribution of the variables was evaluated via the Shapiro-Wilk test. For OF and EPM tests, MWM probe trial, and bio-chemical parameters, normally distributed variables were analyzed via one-way analysis of variance (ANOVA), followed by Tukey’s post hoc honest significant difference (HSD) test. For non-normal variables, Kruskal–Wallis test was performed. Data recorded from the MWM spatial navigation task were analyzed via repeated measures ANOVA, with session as the repeated measure. Correlation of biochemical parameters with MWM probe trail results was analyzed using the Spearman’s correlation coefficient (for BDNF and NGF) and Pearson’s
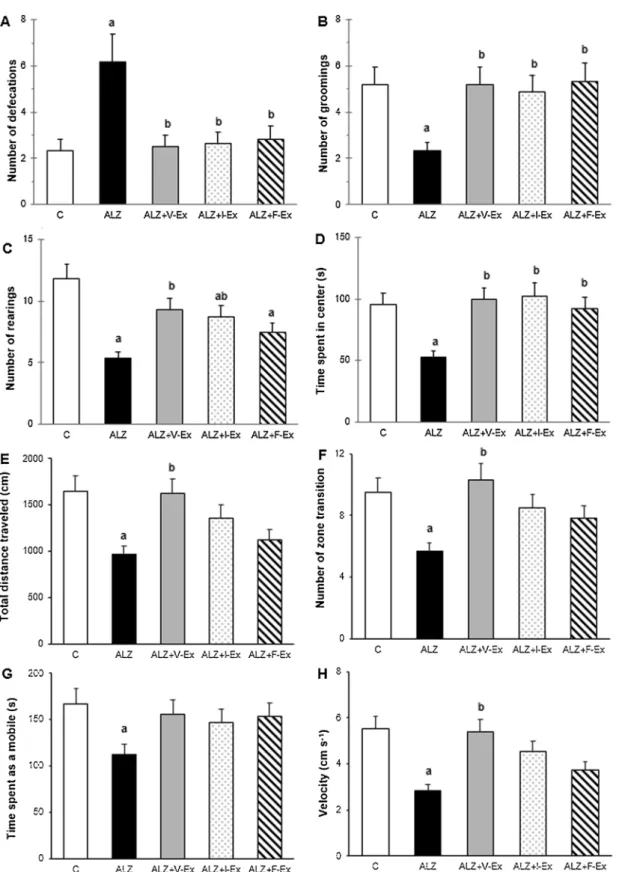
Fig. 2. Effects of different exercise modalities on number of defecations (A), groomings (B), and rearings (C), time spent in the center (D), total distance traveled (E),
number of zone transition (F), time spent as a mobile (G), and velocity (H) during Open Field test of rats which were exposed to D-GAL + AlCl3. Data are expressed as
mean ± SEM.aP < 0.05 compared to the C group andbP < 0.05 compared to the ALZ group. C: Control group, ALZ: Alzheimer model group, ALZ + V-Ex:
correlation (for Aβ1–42, MAPT, MDA, PC, SOD and GSH). Statistical
significance was set at P < 0.05. 3. Results
3.1. Behavioral analysis
3.1.1. Exercise training improves locomotor activity and exploratory behavior in AD pathology
In the OF and EPM tests, total distance traveled and average velocity are indicators of the locomotor activity. Additionally, in the OF appa-ratus, time spent as a mobile is also a measure of locomotor activity. According to the ANOVA analysis, in the OF apparatus, total distance traveled (F4,27= 3.652; P = 0.017) average velocity (F4,27= 5.356;
P = 0.003) and time spent as a mobile (F4,27= 3.358; P = 0.024) were
different between the groups. Total distance traveled (Fig. 2E) and average velocity (Fig. 2H) were lower in the ALZ group than in the C group (P < 0.05, and P < 0.05, respectively), but were higher in the ALZ + V-Ex group than in the ALZ group (P < 0.05, and P < 0.05, respectively). Time spent as a mobile was lower in the ALZ group than in the C group (P < 0.05) (Fig. 2G). According to the ANOVA analysis, in the EPM apparatus, total distance traveled (F4,27= 3.993;
P = 0.011) and average velocity (F4,27= 2.984; P = 0.037) were
dif-ferent between the groups. Total distance traveled was higher in the ALZ + V-Ex (P < 0.05) and ALZ + I-Ex (P < 0.05) groups than in the ALZ group, and average velocity was higher in the ALZ + I-Ex group than in the ALZ group (P < 0.05) (Fig. 3D). These data indicate that locomotor activity is declined with D-GAL and AlCl3administration
especially in the OF test and this impairment is reversed by exercise training in both OF and EPM apparatuses especially with the voluntary
exercise training.
In the OF apparatus, number of rearings and zone transitions are measure of exploratory behavior. According to the ANOVA analysis, number of rearings (F4,27= 10.720; P = 0.000) and zone transitions
(F4,27= 3.883; P = 0.013) were different between the groups. The
number of rearings was lower in the ALZ (P < 0.001), ALZ + I-Ex (P < 0.05), and ALZ + F-Ex (P < 0.05) groups than in the C group, but was higher in the ALZ + V-Ex (P < 0.05), and ALZ + I-Ex (P < 0.05) groups than in the ALZ group (Fig. 2C). The number of zone transition was lower in the ALZ group than in the C group (P < 0.05), but was higher in the ALZ + V-Ex group than in the ALZ group (P < 0.05) (Fig. 2F). These data demonstrate that exploratory behavior is impaired with D-GAL and AlCl3administration and this decline is
reversed by exercise training especially with the voluntary and in-voluntary exercise trainings.
3.1.2. Exercise training attenuates severity of anxiety-like behavior induced by AD pathology
In the OF apparatus, number of defecations, and groomings and time spent in the center are indicators of the anxiety-like behavior. Additionally, in the EPM apparatus, number of entries in closed, and open arms are also a measure of anxiety-like behavior. According to the ANOVA analysis, in the OF apparatus, number of groomings (F4,27= 8.329; P = 0.000), time spent in the center (F4,27= 8.943;
P = 0.000), and the number of defecations (F4,27= 29.876; P = 0.000)
were different between the groups. The number of groomings (Fig. 2B), and time spent in the center (Fig. 2D) were lower in the ALZ group than in the C group (P < 0.001, and P < 0.05, respectively), but were higher in the ALZ + V-Ex (P < 0.001, and P < 0.001, respectively), ALZ + I-Ex (P < 0.001, and P < 0.001, respectively), and ALZ + F-Ex
Fig. 3. Effects of different exercise modalities on total distance traveled (A), number of entries in closed arms (B), and open arms (C) and mean velocity (D) during the
Elevated Plus Maze test of rats which were exposed to D-GAL + AlCl3. Data are expressed as mean ± SEM.aP < 0.05 compared to the C group andbP < 0.05
compared to the ALZ group. C: Control group, ALZ: Alzheimer model group, ALZ + V-Ex: Alzheimer + voluntary exercise model group, ALZ + I-Ex: Alzheimer + involuntary exercise model group, ALZ + F-Ex: Alzheimer + forced exercise model group.
(P < 0.001, and P < 0.05, respectively) groups than in the ALZ group. The number of defecations was higher in the ALZ group than in the C group (P < 0.001), but was lower in the ALZ + V-Ex (P < 0.001), ALZ + I-Ex (P < 0.001), and ALZ + F-Ex (P < 0.001) groups than in the ALZ group (Fig. 2A). According to the ANOVA analysis, in the EPM apparatus, the number of entries in the closed (F4,27= 10.769;
P = 0.000) and open (F4,27= 17.828; P = 0.000) arms were different
between the groups. The number of entries in the closed arms was higher in the ALZ group than in the C group (P < 0.001), but was lower in the ALZ + V-Ex (P < 0.001), ALZ + I-Ex (P < 0.001), and ALZ + F-Ex (P < 0.001) groups than in the ALZ group (Fig. 3B). The number of entries in the open arms was lower in the ALZ group than in the C group (P < 0.001), but was higher in the ALZ + V-Ex (P < 0.001), ALZ + I-Ex (P < 0.001), and ALZ + F-Ex (P < 0.001) groups than in the ALZ group (Fig. 3C). These data indicate that D-GAL and AlCl3administration induces an increase in the anxiety levels and
voluntary, involuntary, and forced exercise trainings attenuate severity of anxiety-like behavior.
3.1.3. Exercise training prevents spatial learning and memory impairment due to AD pathology
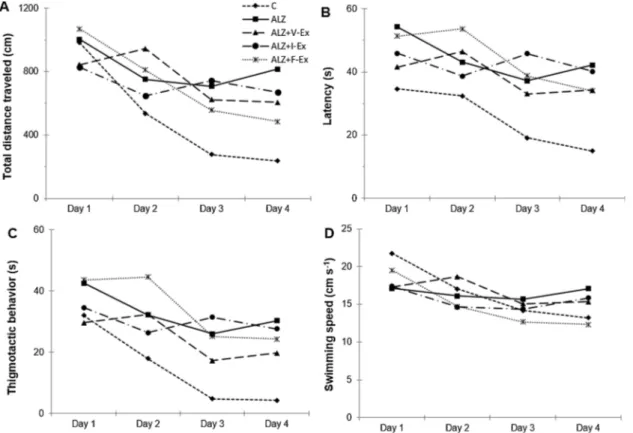
To investigate the effects of different exercise training modalities on D-GAL and AlCl3-induced cognitive impairment, we examined spatial
learning and memory using the MWM test. A repeated measures ANOVA test showed that during the spatial navigation task of the MWM, total distance traveled (F4,27= 27.665; P = 0.000), latency
(F4,27= 13.000; P = 0.000), thigmotactic behavior (F4,27= 21.098;
P = 0.000), and average swimming speed (F4,27= 13.440; P = 0.000)
reduced with time in all groups and did not differ between the groups (P > 0.05) (Fig. 4A–D). These data indicate that rats spatial learning ability improved over the course of training trails in all groups.
Fig. 5shows MWM probe task results of the groups. According to the
ANOVA analysis, total distance traveled (F4,27= 5.232; P = 0.003),
total time spent in the target quadrant (F4,27= 14.163; P = 0.025), the
number of platform crossings (F4,27= 14.851; P = 0.000), and time
spent in the platform zone (F4,27= 47.507; P = 0.000) were different
between the groups. Total distance traveled was lower in the ALZ + I-Ex (P < 0.05), and ALZ + F-I-Ex (P < 0.05) groups than in the C group (Fig. 5A). Total time spent in the SE quadrant (target quadrant) was higher in all groups, as compared to the other quadrants (P < 0.05) (Fig. 5B). The number of platform crossings (Fig. 5C), and time spent in the platform zone (Fig. 5D) were lower in the ALZ group than in the C group (P < 0.001, and P < 0.001, respectively), but were higher in the ALZ + V-Ex (P < 0.001, and P < 0.001, respectively), ALZ + I-Ex (P < 0.001, and P < 0.001, respectively), and ALZ + F-Ex (P < 0.001, and P < 0.001, respectively) groups than in the ALZ group. These data suggest that D-GAL and AlCl3administration impairs
the learning ability of the rats and all exercise training modalities prevent this impairment.
3.2. Biochemical analysis
3.2.1. Exercise training suppresses markers of AD pathology in the hippocampus
Pathologically, D-GAL and AlCl3administration induces
accumula-tion of extracellular aggregates of Aβ proteins in the senile plaques and the intracellular aggregates of hyperphosphorylated tau in the neuro-fibrillary tangles, which are the two pathological hallmarks observed in the AD. Thus, we assessed Aβ and tau pathology by measuring Aβ1–42,
and MAPTlevels in the hippocampus. According to the ANOVA
ana-lysis, Aβ1–42(F4,27= 14.579; P = 0.000), and MAPT (F4,27= 19.469;
P = 0.000) levels were different between the groups. Aβ1–42(Fig. 6A),
and MAPT (Fig. 6B) levels were higher in the ALZ (P < 0.001, and
P < 0.001, respectively), ALZ + V-Ex (P < 0.05, and P < 0.05,
Fig. 4. Effects of different exercise modalities on total distance traveled (A), latency to find the platform (B), thigmotactic behavior (C), and mean swimming speed
(D) during Morris Water Maze spatial navigation task of rats which were exposed to D-GAL + AlCl3. Data are expressed as mean ± SEM. C: Control group, ALZ:
Alzheimer model group, ALZ + V-Ex: Alzheimer + voluntary exercise model group, ALZ + I-Ex: Alzheimer + involuntary exercise model group, ALZ + F-Ex: Alzheimer + forced exercise model group.
respectively), and ALZ + I-Ex (P < 0.05, and P < 0.05, respectively) group than in the C group, but was lower in the ALZ + V-Ex (P < 0.05, and P < 0.001, respectively), ALZ + I-Ex (P < 0.001, and P < 0.001, respectively), and ALZ + F-Ex (P < 0.001, and P < 0.001, respec-tively) groups than in the ALZ group. Additionally, in the hippocampus Aβ1–42and MAPTlevels showed a significant negative correlation with
the number of platform crossing and time spent in the platform zone (Table 1). Collectively, the findings of this study suggest that voluntary, involuntary, or forced exercise training decreases the severity of AD pathology.
3.2.2. Exercise training ameliorates hippocampal neurotrophic factor levels in AD pathology
Neurotrophic factors play important roles in the pathogenesis of AD. Therefore, we evaluated the levels of the neurotrophic factors by measuring NGF and BDNF levels in the hippocampus. According to the ANOVA analysis, NGF (F4,27= 5.715; P = 0.002), and BDNF
(F4,27= 19.469; P = 0.000) levels were different between the groups.
NGF level was higher in the ALZ + V-Ex (P < 0.05), ALZ + I-Ex (P < 0.05), and ALZ + F-Ex (P < 0.05) groups than in the ALZ group (Fig. 7A). BDNF level was higher in the ALZ + F-Ex group than in the ALZ group (P < 0.05) (Fig. 7B). Additionally, hippocampal BDNF and NGF levels demonstrated a significant positive correlation with the number of platform crossing and time spent in the platform zone (Table 1). These data indicate that D-GAL and AlCl3administration
decreases the neurotropic factor levels, but exercise training prevents this decline.
3.2.3. Exercise training improves hippocampal oxidative stress and antioxidant defense in AD pathology
Since, oxidative stress and antioxidant defense system play an
important role in the pathophysiology of AD, we assessed the hippo-campal levels of the MDA, PC, GSH, and SOD. According to the ANOVA analysis, MDA (F4,27= 9.921; P = 0.000), PC (F4,27= 6.782;
P = 0.001) and GSH (F4,27= 6.932; P = 0.001) levels and SOD
activ-ities (F4,27= 6.003; P = 0.001) were different between the groups.
MDA level was higher in the ALZ group than in the C group (P < 0.001), but was lower in the ALZ + V-Ex (P < 0.001), ALZ + I-Ex (P < 0.05), and ALZ + F-I-Ex (P < 0.001) groups than in the ALZ group (Fig. 8A). PC level was higher in the ALZ group than in the C group (P < 0.001), but was lower in the ALZ + I-Ex (P < 0.05), and ALZ + F-Ex (P < 0.001) groups than in the ALZ group (Fig. 8B). GSH level was higher in the ALZ + V-Ex (P < 0.001), and ALZ + I-Ex (P < 0.001) groups than in the ALZ group (Fig. 8C). SOD activity was lower in the ALZ group than in the C group (P < 0.001), but was higher in the ALZ + I-Ex group than in the ALZ group (P < 0.05) (Fig. 8D). Additionally, in the hippocampus MDA and PC levels showed a significant negative correlation and GSH level, and SOD activity showed a significant positive correlation with the number of platform crossing and time spent in the platform zone (Table 1). Collectively, these findings indicate that exercise training reverses D-GAL and AlCl3
administration induced impairment in the hippocampal oxidative stress and antioxidant system.
4. Discussion
In the present study, we examined the impact of different exercise modalities on behavioral parameters and levels of the AD pathology markers, neurotrophic factors, and oxidative damage in the experi-mental AD model induced by D-GAL and AlCl3administration in the
rats. Specifically, we have compared voluntary exercise in a wheel, involuntary exercise in a swimming pool without load or forced
Fig. 5. Effects of different exercise modalities on total distance traveled (A) time spent in each quadrant (B), number of platform crossings (C), and time spent in
platform zone (D) during Morris Water Maze probe task of rats which were exposed to D-GAL + AlCl3. Data are expressed as mean ± SEM.aP < 0.05 compared to
the C group andbP < 0.05 compared to the ALZ group. C: Control group, ALZ: Alzheimer model group, ALZ + V-Ex: Alzheimer + voluntary exercise model group,
exercise in a swimming pool with load. It is noticeable that the all three types of exercise protocols improve behavioral deficits, suppress AD pathology markers, and ameliorate the neurotrophic factors and oxi-dative stress in the experimental AD model.
Consistent with the previous studies [12,49,50] which have been stated that non-cognitive functions are negatively affected at all stages of AD, in the present study, locomotor activity and exploratory beha-vior decreased in the experimental AD model and these impairments were reversed by different exercise modalities. In contrast to our results [9] observed no significant difference between the transgenic TgCRND8 mice and non-Tg littermates in total distance traveled, and total dis-tance of exploration in the OF test. Additionally, in a recent study Bassani et al. [51] observed an increase in locomotor activity, but no differences in exploratory behavior compared with the sham group in the experimental AD model induced with intracerebroventricular streptozotocin (icv-STZ) injection. These discrepancies among the re-sults may be attributable to differences in the animals’ age, gender, and species and time interval between the behavioral tests and differences in the AD induction protocols. The present findings revealed that reg-ular exercise training produced a positive effect on our AD model in the locomotor activity, and exploratory behavior as shown in the OF and EPM tests. Consistent with our findings it has been demonstrated that exercise training ameliorates these non-cognitive symptoms associated with the AD disease [12,52,53].
In the present study, D-GAL and AlCl3administrated rats spent more
time on the closed arms, and less time on the open arms of the EPM and
Fig. 6. Amyloid beta 1–42 (Aβ1–42) and microtubule associated protein Tau
(MAPT) measurements in hippocampal tissue of the rats which were exposed to
D-GAL + AlCl3and which were performed different exercise modalities. Data
are expressed as mean ± SEM. aP < 0.05 compared to the C group and bP < 0.05 compared to the ALZ group. C: Control group, ALZ: Alzheimer
model group, ALZ + V-Ex: Alzheimer + voluntary exercise model group, ALZ + I-Ex: Alzheimer + involuntary exercise model group, ALZ + F-Ex: Alzheimer + forced exercise model group.
Table 1
Correlations between the main indices of the cognitive functions and measured biochemical parameters.
Total distance
traveled The number ofplatform crossings
Time spent in the platform zone Aβ1-42 Correlation (r) −0.172 −0.493 −0.589 Significance (P) 0.346 0.004 0.000 MAPT Correlation (r) −0.121 −0.546 −0.638 Significance (P) 0.511 0.001 0.000 BDNF Correlation (r) −0.185 0.489 0.627 Significance (P) 0.310 0.004 0.000 NGF Correlation (r) −0.274 0.352 0.620 Significance (P) 0.129 0.048 0.000 MDA Correlation (r) 0.000 −0.503 −0.715 Significance (P) 0.999 0.003 0.000 PC Correlation (r) −0.070 −0.435 −0.540 Significance (P) 0.704 0.013 0.001 GSH Correlation (r) −0.336 0.604 0.581 Significance (P) 0.060 0.000 0.000 SOD Correlation (r) 0.120 0.394 0.490 Significance (P) 0.515 0.026 0.004
Aβ1–42: Amyloid beta 1–42, MAPT: Microtubule associated protein Tau, NGF:
Nerve growth factor, BNDF: Brain-derived neurotrophic factor, MDA: Malondialdehyde, PC: Protein carbonyl, GSH: Glutathione, SOD: Superoxide dismutase.
Fig. 7. Nerve growth factor (NGF), and brain-derived neurotrophic factor
(BDNF) levels in hippocampal tissue of the rats which were exposed to D-GAL + AlCl3and which were performed different exercise modalities. Data are
expressed as mean ± SEM.bP < 0.05 compared to the ALZ group. C: Control
group, ALZ: Alzheimer model group, ALZ + V-Ex: Alzheimer + voluntary ex-ercise model group, ALZ + I-Ex: Alzheimer + involuntary exex-ercise model group, ALZ + F-Ex: Alzheimer + forced exercise model group.
spent less time in the center, less groomed and more defecated in the OF, suggesting that these animals were more “anxious” than the control animals. In the previous studies it has been generally demonstrated that AD disease induced anxiety-like behaviors in different AD models such as transgenic animals [54,55], icv-STZ injection [12,55], and D-GAL and AlCl3administration [43]. However, it has been also claimed that
in the experimental AD model, anxiety-like behaviors unchanged [9] or decreased [51] compared to the healthy controls. The differences among the results may be depend on the animals’ age, gender, and species and time interval between the behavioral tests and differences in the AD induction protocols. However, in the present study increased anxiety levels may be attributable to accumulation of Aβ protein and tau. The present findings revealed that voluntary, involuntary, and forced exercises induced a decrease in the anxiety levels and all exercise modalities appeared to equally affects anxiety levels. Consistent with our findings it has been reported that both voluntary [53] and in-voluntary [12,56,57] exercise trainings induced a reduction in the an-xiety levels.
In the present study, behavioral data showed a cognitive decline in the MWM after the 90 days of D-GAL and AlCl3administration. It has
been well known that AD disease induced spatial memory impairment and this situation has been demonstrated via different experimental models including transgenic animals [22], icv-STZ injection [58], and D-GAL and AlCl3 administration [43,59]. In the present study the
neuroprotective effect of different exercise modalities was clearly ob-served during the MWM probe trial via a significantly high number of platform crossings and time spent in the platform zone. These data are consistent with other studies [6,23,60] showing that exercise training can reverse experimental AD-induced spatial learning and memory deficit. In this study, voluntary, involuntary, and forced exercise trainings seemed to almost equally affects spatial memory. To the best of our knowledge this is the first study comparing the efficiency of
voluntary, involuntary, and forced exercise trainings in the AD pa-thology. However, in a recent study, Diederich et al. [61] compared to the effects voluntary, and involuntary exercise trainings on cognitive functions with MWM in obese mice. Consistent with our findings they showed that both voluntary and involuntary exercise trainings improve the acquisition and retention of spatial memory.
Accumulation of Aβ protein and tau hyperphosphorylation in the hippocampus is responsible for the behavioral deficiency observed in both AD patients and experimental AD models ([62,63]. In line with this findings, in the present study hippocampal Aβ1–42and MAPTlevels
were higher in the D-GAL and AlCl3administrated rats when compared
to the control rats. Consistent with our results it has been showed that D-GAL and AlCl3administration induced an increase in the Aβ and tau
protein levels [43]. In the present study different exercise modalities induced a general reduction in Aβ and tau pathology in AD-induced rats in agreement with the previous studies [23,29,43]. However, forced exercise seems to be less effective on AD pathology markers compared to the voluntary and involuntary exercises. This results indicate that exercise intensity is a key element for the beneficial effects of exercise training. Beneficial effects of exercise training on the hippocampal Aβ and tau levels has been demonstrated in the different experimental AD models such as transgenic animals [23,64], icv-STZ injection [8], and D-GAL and AlCl3administration [43]. Both treadmill running and
vo-luntary wheel running protocols have been consistently found to reduce AD pathology in the rodents. They both reduce Aβ plaque number, soluble Aβ protein and tau phosphorylation [6].
In this study, hippocampal BDNF and NGF levels tended to be lower in the experimental AD model when compared to the healthy controls. Previous studies that utilized different AD models in rodents including transgenic animals [65], icv-STZ injection [66], and D-GAL and AlCl3
administration [67] showed a reduction in hippocampal BDNF and/or NGF levels accompanied by behavioral impairment. Additionally, in AD
Fig. 8. Malondialdehyde (MDA), protein carbonyl (PC), glutathione (GSH), and superoxide dismutase (SOD) activity measurements in hippocampal tissue of the rats
which were exposed to D-GAL + AlCl3and which were performed different exercise modalities. Data are expressed as mean ± SEM.aP < 0.05 compared to the C
group andbP < 0.05 compared to the ALZ group. C: Control group, ALZ: Alzheimer model group, ALZ + V-Ex: Alzheimer + voluntary exercise model group,
patients, it has been found that circulating BDNF levels [68] and hip-pocampal BDNF expressions [69] were lower than healthy subjects. In this study statistically non-significant decrease in the BDNF and NGF levels might be depend on the small sample size. In the present study voluntary, involuntary and forced exercises induced an increase in the NGF levels. However, although voluntary, involuntary and forced ex-ercises also induced an increase in the BDNF levels, only increase in the forced exercise groups was statically significant. This situation might be depending on the small sample size. It has been generally known that exercise training increases both expression and level of hippocampal BDNF and NGF, and through their neuroprotective effects improve behavioral impairment in AD [70]. Nigam et al. [29] claimed that vo-luntary exercise training reduced Aβ levels via increasing hippocampal BDNF expression. Additionally, in a recent study Azimi et al. [35] showed that long-term treadmill running prevents AD-induced decrease in BDNF levels. Taken together these data indicate that increasing the levels of neurotrophic factors, exercise training could mediate the im-provement in the behavioral functions in AD.
In the present study, experimental AD-induced rats with D-GAL and AlCl3administration suffer from the hippocampal oxidative stress with
increased MDA and PC levels, and reduced GSH level and SOD activity when compared to the controls. It has been suggested that AD devel-opment is a direct consequence of the increase of oxidative stress in the brain [71]. Consistent with our findings many studies [64,58,59] showed an increase in oxidative stress and decrease in antioxidant de-fense system in experimental AD model. In the present study, voluntary, involuntary and forced exercise trainings made possible the restoration of the oxidative stress and antioxidant defense via decreasing the MDA and PC levels and increasing the GSH level and SOD activity. These findings are in line with previous studies [72,64,73] in which a di-minution in oxidative stress and improvement in antioxidant defense has been observed in different AD models by using different exercise modalities. It has been reported that endogenous non-enzymatic anti-oxidant defense markers such as GSH is significantly enhanced after exercise training in the brains of the experimental AD models [58,74]. However, although small differences observed, all exercise modalities seemed to almost equally affects oxidative stress and antioxidant de-fense system.
This study has several limitations that should be underlined. One limitation of our study is that during voluntary exercise, the rats were single housed and this situation could be stressful for the animals. Another limitation is that we did not use molecular techniques such as, quantitative polymerase chain reaction (qPCR) or Western Blotting due to the technical difficulties. Additional research is needed to identify the mechanism of action of the different effect modalities at the mo-lecular level and further clarify the relationship between markers of the AD pathology, oxidative stress, and neurotrophic factors.
Taken together, our results showed that voluntary, involuntary and forced exercise trainings almost equally attenuated non-cognitive dis-turbances and cognitive dysfunction in the experimental AD model induced by D-GAL and AlCl3administration in the rats. Suppression of
AD pathology markers, elevated neurotrophic factor levels, and im-proved oxidative stress and antioxidant defense system could mediate the beneficial effects of different exercise modalities.
Conflict of interest statement
The authors declare there are no conflicts of interest—financial or otherwise—related to the material presented herein.
References
[1] R. Anand, K.D. Gill, A.A. Mahdi, Therapeutics of Alzheimer’s disease: past, present and future, Neuropharmacology 76 (January (Pt A)) (2014) 27–50.
[2] K. Kumar, A. Kumar, R.M. Keegan, R. Deshmukh, Recent advances in the neuro-biology and neuropharmacology of Alzheimer’s disease, Biomed. Pharmacother. 98
(February) (2018) 297–307.
[3] J.H. Koo, I.S. Kwon, E.B. Kang, C.K. Lee, N.H. Lee, M.G. Kwon, I.H. Cho, J.Y. Cho, Neuroprotective effects of treadmill exercise on BDNF and PI3-K/Akt signaling pathway in the cortex of transgenic mice model of Alzheimer’s disease, J. Exerc. Nutrition Biochem. 17 (December (4)) (2013) 151–160.
[4] J. Viña, C. Borras, K.M. Abdelaziz, R. Garcia-Valles, M.C. Gomez-Cabrera, The free radical theory of aging revisited: the cell signaling disruption theory of aging, Antioxid. Redox Signal. 19 (2013) 779–787.
[5] E. Tönnies, E. Trushina, Oxidative stress, synaptic dysfunction, and Alzheimer’s disease, J. Alzheimers Dis. 57 (4) (2017) 1105–1121.
[6] S.M. Ryan, ÁM. Kelly, Exercise as a pro-cognitive, pro-neurogenic and anti-in-flammatory intervention in transgenic mouse models of Alzheimer’s disease, Ageing Res. Rev. 27 (May) (2016) 77–92.
[7] E.J. Mufson, L. Mahady, D. Waters, S.E. Counts, S.E. Perez, S.T. DeKosky, S.D. Ginsberg, M.D. Ikonomovic, S.W. Scheff, L.I. Binder, Hippocampal plasticity during the progression of Alzheimer’s disease, Neuroscience 309 (November) (2015) 51–67.
[8] Y. Lu, Y. Dong, D. Tucker, R. Wang, M.E. Ahmed, D. Brann, Q. Zhang, Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a streptozotocin-induced rat model of sporadic Alzheimer’s disease, J. Alzheimers Dis. 56 (4) (2017) 1469–1484.
[9] E. Maliszewska-Cyna, K. Xhima, I. Aubert, A comparative study evaluating the impact of physical exercise on disease progression in a mouse model of Alzheimer’s disease, J. Alzheimers Dis. 53 (May(1)) (2016) 243–257.
[10] N.J. Haughey, A. Nath, S.L. Chan, A.C. Borchard, M.S. Rao, M.P. Mattson, Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural pro-genitor cell homeostasis, in models of Alzheimer’s disease, J. Neurochem. 83 (December (6)) (2002) 1509–1524.
[11] R. Hoveida, H. Alaei, S. Oryan, K. Parivar, P. Reisi, Treadmill running improves spatial memory in an animal model of Alzheimer’s disease, Behav. Brain Res. 216 (January (1)) (2011) 270–274.
[12] A.T. Dao, M.A. Zagaar, S. Salim, J.L. Eriksen, K.A. Alkadhi, Regular exercise pre-vents non-cognitive disturbances in a rat model of Alzheimer’s disease, Int. J. Neuropsychopharmacol. 17 (April (4)) (2014) 593–602.
[13] G. Leal, C.R. Bramham, C.B. Duarte, BDNF and hippocampal synaptic plasticity, Vitam. Horm. 104 (2017) 153–195.
[14] K.A. Intlekofer, C.W. Cotman, Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease, Neurobiol. Dis. 57 (September) (2013) 47–55. [15] R.E.K. MacPherson, Filling the void: a role for exercise-induced BDNF and brain amyloid precursor protein processing, Am. J. Physiol. Regul. Integr. Comp. Physiol. 313 (November (5)) (2017) R585–R593.
[16] F. Fumagalli, G. Racagni, M.A. Riva, The expanding role of BDNF: a therapeutic target for Alzheimer’s disease? Pharmacogenomics J. 6 (January–February (1)) (2006) 8–15.
[17] L. Zhang, Y. Fang, Y. Lian, Y. Chen, T. Wu, Y. Zheng, H. Zong, L. Sun, R. Zhang, Z. Wang, Y. Xu, Brain-derived neurotrophic factor ameliorates learning deficits in a rat model of Alzheimer’s disease induced by aβ1-42, PLoS One (April (4)) (2015) e0122415.
[18] C. Hock, K. Heese, C. Hulette, C. Rosenberg, U. Otten, Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas, Arch. Neurol. 57 (June (6)) (2000) 846–851.
[19] S. Arancibia, M. Silhol, F. Moulière, J. Meffre, I. Höllinger, T. Maurice, L. Tapia-Arancibia, Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats, Neurobiol. Dis. 31 (September (3)) (2008) 316–326. [20] C. Matrone, M.T. Ciotti, D. Mercanti, R. Marolda, P. Calissano, NGF and BDNF
signaling control amyloidogenic route and Abeta production in hippocampal neu-rons, Proc. Natl. Acad. Sci. U. S. A. 105 (September (35)) (2008) 13139–13144. [21] E. Duzel, H. van Praag, M. Sendtner, Can physical exercise in old age improve
memory and hippocampal function? Brain 139 (March (Pt 3)) (2016) 662–673. [22] J. Cho, M.K. Shin, D. Kim, I. Lee, S. Kim, H. Kang, Treadmill running reverses
cognitive declines due to alzheimer disease, Med. Sci. Sports Exerc. 47 (September (9)) (2015) 1814–1824.
[23] J.H. Koo, E.B. Kang, Y.S. Oh, D.S. Yang, J.Y. Cho, Treadmill exercise decreases amyloid-β burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer’s disease, Exp. Neurol. 288 (February) (2017) 142–152.
[24] K.M. Moore, R.E. Girens, S.K. Larson, M.R. Jones, J.L. Restivo, D.M. Holtzman, J.R. Cirrito, C.M. Yuede, S.D. Zimmerman, B.F. Timson, A spectrum of exercise training reduces soluble Aβ in a dose-dependent manner in a mouse model of Alzheimer’s disease, Neurobiol. Dis. 85 (January) (2016) 218–224. [25] X. Jiang, G.S. Chai, Z.H. Wang, Y. Hu, X.G. Li, Z.W. Ma, Q. Wang, J.Z. Wang,
G.P. Liu, CaMKII-dependent dendrite ramification and spine generation promote spatial training-induced memory improvement in a rat model of sporadic Alzheimer’s disease, Neurobiol. Aging 36 (February (2)) (2015) 867–876. [26] S. Revilla, C. Suñol, Y. García-Mesa, L. Giménez-Llort, C. Sanfeliu, R. Cristòfol,
Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain, Neuropharmacology 81 (June) (2014) 55–63. [27] H.S. Um, E.B. Kang, J.H. Koo, H.T. Kim, Kim E.J. Jin-Lee, C.H. Yang, G.Y. An, I.H. Cho, J.Y. Cho, Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease, Neurosci. Res. 69 (February (2)) (2011) 161–173.
[28] H.C. Ke, H.J. Huang, K.C. Liang, H.M. Hsieh-Li, Selective improvement of cognitive function in adult and aged APP/PS1 transgenic mice by continuous non-shock treadmill exercise, Brain Res. 27 (July(1403)) (2011) 1–11.
[29] S.M. Nigam, S. Xu, J.S. Kritikou, K. Marosi, L. Brodin, M.P. Mattson, Exercise and BDNF reduce Aβ production by enhancing α-secretase processing of APP, J.
Neurochem. 142 (July (2)) (2017) 286–296.
[30] Y. García-Mesa, H. Pareja-Galeano, V. Bonet-Costa, S. Revilla, M.C. Gómez-Cabrera, J. Gambini, L. Giménez-Llort, R. Cristòfol, J. Viña, C. Sanfeliu, Physical exercise neuroprotects ovariectomized 3xTg-AD mice through BDNF mechanisms, Psychoneuroendocrinology 45 (July) (2014) 154–166.
[31] D. Özbeyli, G. Sarı, N. Özkan, B. Karademir, M. Yüksel, ÖT Çilingir Kaya, Ö. Kasımay Çakır, Protective effects of different exercise modalities in an Alzheimer’s disease-like model, Behav. Brain Res. 328 (June) (2017) 159–177. [32] Z. Zhang, H. Wu, H. Huang, Epicatechin plus treadmill exercise are neuroprotective
against moderate-stage amyloid precursor Protein/Presenilin 1 mice, Pharmacogn. Mag. 12 (May (Suppl 2)) (2016) S139–S146.
[33] L.C. Souza, C.R. Jesse, L. Del Fabbro, M.G. de Gomes, A.T.R. Goes, C.B. Filho, C. Luchese, A.A.M. Pereira, S.P. Boeira, Swimming exercise prevents behavioural disturbances induced by an intracerebroventricular injection of amyloid-β(1-42) peptide through modulation of cytokine/NF-kappaB pathway and indoleamine-2,3-dioxygenase in mouse brain, Behav. Brain Res. 331 (July (28)) (2017) 1–13. [34] M.A. Alomari, O.F. Khabour, K.H. Alzoubi, M.A. Alzubi, Forced and voluntary
ex-ercises equally improve spatial learning and memory and hippocampal BDNF levels, Behav. Brain Res. 15 (June (247)) (2013) 34–39.
[35] M. Azimi, R. Gharakhanlou, N. Naghdi, D. Khodadadi, S. Heysieattalab, Moderate treadmill exercise ameliorates amyloid-β-induced learning and memory impair-ment, possibly via increasing AMPK activity and up-regulation of the PGC-1α/ FNDC5/BDNF pathway, Peptides 102 (April) (2018) 78–88.
[36] S.H. Song, Y.S. Jee, I.G. Ko, S.W. Lee, Y.J. Sim, D.Y. Kim, S.J. Lee, Y.S. Cho, Treadmill exercise and wheel exercise improve motor function by suppressing apoptotic neuronal cell death in brain inflammation rats, J. Exerc. Rehabil. 14 (December (6)) (2018) 911–919.
[37] N. Uysal, M. Kiray, A.R. Sisman, U.M. Camsari, C. Gencoglu, B. Baykara, C. Cetinkaya, I. Aksu, Effects of voluntary and involuntary exercise on cognitive functions, and VEGF and BDNF levels in adolescent rats, Biotech. Histochem. 90 (January (1)) (2015) 55–68.
[38] S. Yanagita, S. Amemiya, S. Suzuki, I. Kita, Effects of spontaneous and forced running on activation of hypothalamic corticotropin-releasing hormone neurons in rats, Life Sci. 80 (January (4)) (2007) 356–363.
[39] M. Ploughman, S. Granter-Button, G. Chernenko, B.A. Tucker, K.M. Mearow, D. Corbett, Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia, Neuroscience 136 (4) (2005) 991–1001.
[40] E. Timofeeva, Q. Huang, D. Richard, Effects of treadmill running on brain activation and the corticotropin-releasing hormone system, Neuroendocrinology 77 (June(6)) (2003) 388–405.
[41] E. Narath, M. Skalicky, A. Viidik, Voluntary and forced exercise influence the sur-vival and body composition of ageing male rats differently, Exp. Gerontol. 36 (November(10)) (2001) 1699–1711.
[42] N. Grissom, S. Bhatnagar, Habituation to repeated stress: get used to it, Neurobiol. Learn. Mem. 92 (September (2)) (2009) 215–224.
[43] H. Li, T. Kang, B. Qi, L. Kong, Y. Jiao, Y. Cao, J. Zhang, J. Yang, Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3 inducing rats model of Alzheimer’s disease, J. Ethnopharmacol. 17 (February (179)) (2016) 162–169.
[44] M.M. Mesulam, A plasticity-based theory of the pathogenesis of Alzheimer’s dis-ease, Ann. N. Y. Acad. Sci. 924 (2000) 42–52.
[45] P. Sengupta, The laboratory rat: relating its age with human’s, Int. J. Prev. Med. 4 (June (6)) (2013) 624–630.
[46] X.M. Peng, L. Gao, S.X. Huo, X.M. Liu, M. Yan, The mechanism of memory en-hancement of acteoside (Verbascoside) in the senescent mouse model induced by a combination of D-gal and AlCl3, Phytother. Res. 29 (August (8)) (2015) 1137–1144. [47] Z. Li, G. Zhao, S. Qian, Z. Yang, X. Chen, J. Chen, C. Cai, X. Liang, J. Guo,
Cerebrovascular protection of β-asarone in Alzheimer’s disease rats: a behavioral, cerebral blood flow, biochemical and genic study, J. Ethnopharmacol. 144 (November (2)) (2012) 305–312.
[48] O.H. Lowry, N.J. Rosebrough, A.L. Farr, R.J. Randall, Protein measurement with the Folin phenol reagent, J. Biol. Chem. 193 (1951) 265–275.
[49] M. Ishii, C. Iadecola, Metabolic and non-cognitive manifestations of Alzheimer’s disease: the hypothalamus as both culprit and target of pathology, Cell Metab. 22 (November (5)) (2015) 761–776.
[50] F. Raudino, Non-cognitive symptoms and related conditions in the Alzheimer’s disease: a literature review, Neurol. Sci. 34 (August (8)) (2013) 1275–1282. [51] T.B. Bassani, J.M. Bonato, M.M.F. Machado, V. Cóppola-Segovia, E.L.R. Moura,
S.M. Zanata, Oliveira RMMW, Vital MABF, Decrease in adult neurogenesis and neuroinflammation are involved in spatial memory impairment in the streptozo-tocin-induced model of sporadic Alzheimer’s disease in rats, Mol. Neurobiol. 55 (May(5)) (2018) 4280–4296.
[52] K.A. Alkadhi, Exercise as a positive modulator of brain function, Mol. Neurobiol. 55 (April (4)) (2018) 3112–3130.
[53] Y. García-Mesa, L. Giménez-Llort, L.C. López, C. Venegas, R. Cristòfol, G. Escames, D. Acuña-Castroviejo, C. Sanfeliu, Melatonin plus physical exercise are highly
neuroprotective in the 3xTg-AD mouse, Neurobiol. Aging 33 (June(6)) (2012) 1124 e13-29.
[54] N.S. Pentkowski, L.E. Berkowitz, S.M. Thompson, E.N. Drake, C.R. Olguin, B.J. Clark, Anxiety-like behavior as an early endophenotype in the TgF344-AD rat model of Alzheimer’s disease, Neurobiol. Aging 61 (January) (2018) 169–176. [55] Y. Chen, Z. Liang, J. Blanchard, C.L. Dai, S. Sun, M.H. Lee, I. Grundke-Iqbal,
K. Iqbal, F. Liu, C.X. Gong, A non-transgenic mouse model (icv-STZ mouse) of Alzheimer’s disease: similarities to and differences from the transgenic model (3xTg-AD mouse), Mol. Neurobiol. 47 (April (2)) (2013) 711–725.
[56] B.K. Kim, M.S. Shin, C.J. Kim, S.B. Baek, Y.C. Ko, Y.P. Kim, Treadmill exercise improves short-term memory by enhancing neurogenesis in amyloid beta-induced Alzheimer disease rats, J. Exerc. Rehabil. 10 (February (1)) (2014) 2–8. [57] L.C. Souza, C.B. Filho, A.T. Goes, L.D. Fabbro, M.G. de Gomes, L. Savegnago,
M.S. Oliveira, C.R. Jesse, Neuroprotective effect of physical exercise in a mouse model of Alzheimer’s disease induced by β-amyloid₁−₄₀ peptide, Neurotox. Res. 24 (August (2)) (2013) 148–163.
[58] L. Rodrigues, M.F. Dutra, J. Ilha, R. Biasibetti, A. Quincozes-Santos, M.C. Leite, S. Marcuzzo, M. Achaval, C.A. Gonçalves, Treadmill training restores spatial cog-nitive deficits and neurochemical alterations in the hippocampus of rats submitted to an intracerebroventricular administration of streptozotocin, J. Neural Transm. Vienna (Vienna) 117 (November (11)) (2010) 1295–1305.
[59] T. Ali, H. Badshah, T.H. Kim, M.O. Kim, Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK signaling pathway in aging mouse model, J. Pineal Res. 58 (January(1)) (2015) 71–85.
[60] M. Hüttenrauch, A. Brauß, A. Kurdakova, H. Borgers, F. Klinker, D. Liebetanz, G. Salinas-Riester, J. Wiltfang, H.W. Klafki, O. Wirths, Physical activity delays hippocampal neurodegeneration and rescues memory deficits in an Alzheimer disease mouse model, Transl. Psychiatry 3 (May (6)) (2016) e800. [61] K. Diederich, A. Bastl, H. Wersching, A. Teuber, J.K. Strecker, A. Schmidt,
J. Minnerup, W.R. Schäbitz, Effects of different exercise strategies and intensities on memory performance and neurogenesis, Front. Behav. Neurosci. 16 (March (11)) (2017) 47.
[62] K. Ebrahimi, A. Majdi, B. Baghaiee, S.H. Hosseini, S. Sadigh-Eteghad, Physical ac-tivity and beta-amyloid pathology in Alzheimer’s disease: a sound mind in a sound body, EXCLI J. 28 (June (16)) (2017) 959–972.
[63] M.P. Mattson, Pathways towards and away from Alzheimer’s disease, Nature 430 (August (7000)) (2004) 631–639.
[64] Y. García-Mesa, S. Colie, R. Corpas, R. Cristòfol, F. Comellas, A.R. Nebreda, L. Giménez-Llort, C. Sanfeliu, Oxidative stress is a central target for physical ex-ercise neuroprotection against pathological brain aging, J. Gerontol. A Biol. Sci. Med. Sci. 71 (January (1)) (2016) 40–49.
[65] M.Y. Liu, S. Wang, W.F. Yao, Z.J. Zhang, X. Zhong, L. Sha, M. He, Z.H. Zheng, M.J. Wei, Memantine improves spatial learning and memory impairments by reg-ulating NGF signaling in APP/PS1 transgenic mice, Neuroscience 273 (July) (2014) 141–151.
[66] Y.J. Sim, Treadmill exercise alleviates impairment of spatial learning ability through enhancing cell proliferation in the streptozotocin-induced Alzheimer’s disease rats, J. Exerc. Rehabil. 10 (April (2)) (2014) 81–88.
[67] Y.S. Abulfadl, N.N. El-Maraghy, A.A.E. Ahmed, S. Nofal, O.A. Badary, Protective effects of thymoquinone on D-galactose and aluminum chloride induced neuro-toxicity in rats: biochemical, histological and behavioral changes, Neurol. Res. 40 (April (4)) (2018) 324–333.
[68] C. Laske, E. Stransky, T. Leyhe, G.W. Eschweiler, K. Schott, H. Langer, M. Gawaz, Decreased brain-derived neurotrophic factor (BDNF)- and beta-thromboglobulin (beta-TG)- blood levels in Alzheimer’s disease, Thromb. Haemost. 96 (July (1)) (2006) 102–103.
[69] G.J. Siegel, N.B. Chauhan, Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain, Brain Res. Brain Res. Rev. 33 (September (2-3)) (2000) 199–227. [70] K.I. Erickson, D.L. Miller, K.A. Roecklein, The aging hippocampus: interactions
between exercise, depression, and BDNF, Neuroscientist 18 (February (1)) (2012) 82–97.
[71] E. Rojas-Gutierrez, G. Muñoz-Arenas, S. Treviño, B. Espinosa, R. Chavez, K. Rojas, G. Flores, A. Díaz, J. Guevara, Alzheimer’s disease and metabolic syndrome: a link from oxidative stress and inflammation to neurodegeneration, Synapse 26 (June) (2017),https://doi.org/10.1002/syn.21990[Epub ahead of print].
[72] T.C. Bernardo, I. Marques-Aleixo, J. Beleza, P.J. Oliveira, A. Ascensão, J. Magalhães, Physical exercise and brain mitochondrial fitness: the possible role against Alzheimer’s disease, Brain Pathol. 26 (September (5)) (2016) 648–663. [73] Z. Radák, T. Kaneko, S. Tahara, H. Nakamoto, J. Pucsok, M. Sasvári, C. Nyakas,
S. Goto, Regular exercise improves cognitive function and decreases oxidative da-mage in rat brain, Neurochem. Int. 38 (January (1)) (2001) 17–23.
[74] H.S. Um, E.B. Kang, Y.H. Leem, I.H. Cho, C.H. Yang, K.R. Chae, D.Y. Hwang, J.Y. Cho, Exercise training acts as a therapeutic strategy for reduction of the pa-thogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model, Int. J. Mol. Med. 22 (October (4)) (2008) 529–539.