Contents lists available atScienceDirect
Computational Biology and Chemistry
journal homepage:www.elsevier.com/locate/cbacResearch Article
Multi-targeted potential of Pittosporum senacia Putt.: HPLC-ESI-MS
n
analysis,
in silico docking, DNA protection, antimicrobial, enzyme inhibition,
anti-cancer and apoptotic activity
Mohamad Fawzi Mahomoodally
a,⁎, Carene Picot-Allain
a, M. Hosenally
b, Asli Ugurlu
c,⁎,
Adriano Mollica
d, Azzurra Stefanucci
d, E.J. Llorent-Martínez
e, Mehmet Cengiz Baloglu
f,
Gokhan Zengin
g,⁎aDepartment of Health Sciences, Faculty of Science, University of Mauritius, 230 Réduit, Mauritius
bDepartment of Economics and Statistics, Faculty of Social Studies & Humanities, University of Mauritius, Réduit, Mauritius cDepartment of Biology, Faculty of Science and Arts, Kastamonu University, Kastamonu, Turkey
dDepartment of Pharmacy, University“G. d’Annunzio” of Chieti-Pescara, 66100 Chieti, Italy eDepartment of Physical and Analytical Chemistry, University of Jaén, Campus Las, Spain
fDepartment of Genetics and Bioengineering, Faculty of Engineering and Architecture, Kastamonu University, Kastamonu, Turkey gDepartment of Biology, Faculty of Science, Selcuk University, Turkey
A R T I C L E I N F O Keywords: Pittosporaceae Mauritius Diabetes Antimicrobial Apoptosis A B S T R A C T
Pittosporum senacia (PS) Putt. (Pittosporaceae), indigenous to the Mascarene Islands, is a common ingredient in traditional medicines. However, there is currently a dearth of studies to validate some of these traditional claims. Given the broad traditional uses of PS against several diseases, we aimed to provide a comprehensive insight into the biological and chemical profile of P. senacia. The antioxidant, enzyme inhibitory activity, anticancer, and phytochemical composition of the methanolic extract of P. senacia leaf extracts were studied. The possible in-teraction and binding mode of the most abundant phytochemicals were studied via in silico docking experiments on tyrosinase andα-glucosidase. The mechanism behind the cytotoxic property of P. senacia extract for MDA-MB-231 was also examined using different methods including 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylte-trazolium bromide (MTT) cell viability test checking apoptosis-associated genes, and wound healing assays. Twenty-six compounds were identified, of which caffeoylquinic acid derivatives, ferulic acid derivative, cin-namoylquinic acid derivative and two other polyphenols (oleuropeine and isoramnetin glucoside) being abun-dant, have been tested using in silico studies, againstα-glucosidase and tyrosinase. The extract (IC50= 118.8μg/
ml) exhibited time and dose dependent anti-proliferative effect on human breast cancer cell line, MDA-MB-231. According to the expression profile of apoptosis inhibitors and apoptosis promoters genes, expression of Bax and Bak genes were significantly increased compared to Bcl-2 and Birc5 genes. Based on wound healing analysis, cell migration was inhibited after the application of the plant extract. The presentfindings suggested that PS might be a good candidate as sources of bioactive compounds for designing functional applications.
1. Introduction
It is widely acknowledge that traditional used medicinal plants possess interesting therapeutic properties used by man since time im-memorial (Aziz et al., 2014;Saleem et al., 2013). Indeed, secondary metabolites, also known as phytochemicals, ubiquitously present in plants possessing tremendous biological potential, are now the
mainstay of drug development (Andrade et al., 2019;Dutt et al., 2019; Rengasamy et al., 2019). The role of medicinal plants as therapeutic tool has become a topic of global importance (Zengin et al., 2018). Substantial amount of scientific investigations have been reported the multitude pharmacological applications of plants extracts and com-pounds isolated from plants extracts. Harnessing the biological poten-tial of medicinal plants represents a sterling opportunity for the
https://doi.org/10.1016/j.compbiolchem.2019.107114
Received 28 July 2019; Received in revised form 20 August 2019; Accepted 23 August 2019
⁎Corresponding authors.
E-mail addresses:f.mahomoodally@uom.ac.mu(M.F. Mahomoodally),picotcarene@yahoo.com(C. Picot-Allain),m.hosenally@uom.ac.mu(M. Hosenally),
a.z.ugurlu@gmail.com(A. Ugurlu),a.mollica@unich.it(A. Mollica),a.stefanucci@unich.it(A. Stefanucci),ellorent@ujaen.es(E.J. Llorent-Martínez),
mcbaloglu@gmail.com(M.C. Baloglu),gokhanzengin@selcuk.edu.tr(G. Zengin).
Available online 29 August 2019
1476-9271/ © 2019 Elsevier Ltd. All rights reserved.
development of novel therapeutic candidates (Ayaz et al., 2019;Maher, 2019;Oyenihi and Smith, 2019).
Pittosporum senacia (PS), belonging to the Pittosporaceae family, is a glabrous, ramified shrub, naturally occurring in Islands of the Indian Ocean, namely, Madagascar, Mauritius, Seychelles, and Réunion Island. Common vernacular names include,“Bois de mangue marron”, “Bois de jolie coeur”, “Bois carottes”, and “Bois malabar”. Ethnobotanical evidence highlights several usage of PS in folk medicine (Suroowan et al., 2019). A decoction was used as purgative, against fever, and amenorrhea. A decoction prepared from PS roots was utilized against nervousness. P. senacia was also reported to manage childhood ailments and also used to treat and/or manage rheumatism, diabetes, syphilis, asthma, and as astringent (Linnek et al., 2012; Suroowan et al., 2019). The crude aqueous and methanolic PS extracts were reported to have anti-microbial activity against both gram-negative and positive bacteria (Mahomoodally et al., 2010). However, there is still a dearth of scien-tific data regarding the biological activities and the phytochemical profile of PS. Given the broad traditional uses of PS against several diseases, we aimed to provide a comprehensive insight into the biolo-gical and chemical profile of PS. The antioxidant, enzyme inhibitory, and phytochemical profile of PS methanolic leaves extract was estab-lished. Moreover, in silico molecular docking studies were performed to elucidate the molecular interactions of some relevant identified com-pounds with the studied enzymes. Biological activities of PS were evaluated in terms of antimicrobial, DNA protective and anticancer activity. Cytotoxicity of PS was tested on breast cancer cells in vitro and underlying molecular mechanisms were further analyzed.
2. Material and methods 2.1. Plant material
The plant samples were collected at Pétrin, Mauritius in 2018. The sample was identified by the Herbarium of Mauritius Sugar Industry Research Institute. Freshly collected leaves were weighed, thoroughly washed and patted dry before allowed to air dry until a constant mass was reached. The dried leaves were then grinded tofine homogenized powder and stored in clean McCartney vials at 4 °C. To prepare me-thanolic extracts, 50 g of leaf powder were added to an Erlenmeyer flask followed by 500 mL of methanol. The flasks were wrapped in cellulose paper and covered with aluminum foil to protect the samples. The samples were allowed to macerate in the solvents forfive days on a magnetic stirrer with constant mixing and thenfiltrated using Whatman (Grade 1) paper. The solvents in the collected filtrate were dried in vacuo. The collected extracts were then placed in clean vials and stored at 4 °C.
2.2. Instrumentation
We used an Agilent Series 1100 HPLC system with a G1315B diode array detector (Agilent Technologies). Separation was performed in a Luna Omega Polar C18analytical column (150 × 3.0 mm; 5μm particle size) with a Polar C18 Security Guard cartridge (4 × 3.0 mm) from Phenomenex. An ion trap mass spectrometer (Esquire 6000, Bruker Daltonics) with an electrospray interface was connected to the HPLC-system. Detailed HPLC-MS conditions are available in the study of Llorent-Martínez et al. (2018). For chromatographic analyses, 10μL of sample (5 mg mL−1DE in MeOH) was injected.
2.3. Profile of total bioactive compounds
By referring to our previous paper (Uysal et al., 2017b), the flavo-noids (TFC) and total phenolic (TPC) contents were determined using the AlCl3 and Folin-Ciocalteu assays, respectively. The results were expressed as equivalents of rutin (mg RE/g) for TFC and gallic acid (mg GAE/g) for TPC.
2.4. Antioxidant and enzyme inhibitory effects
The in vitro enzyme inhibitory effects of extracts on five enzymes, namely, α-amylase, α-glucosidase, acetyl cholinesterase (AChE), bu-tyryl cholinesterase (BChE), and tyrosinase were evaluated, as pre-viously reported (Uysal et al., 2017b). The enzyme inhibitory actions of extracts were assessed as equivalents of kojic acid (KAE) for tyrosinase, galantamine (GALAE) for acetyl and butyryl cholinesterase, and acar-bose (ACAE) forα-amylase and α-glucosidase.
Regarding antioxidant capacity of the extracts, different spectro-photometric experiments as ferrous ion chelating, phosphomo-lybdenum, reducing power (FRAP (ferric reducing antioxidant power) and CUPRAC (cupric reducing antioxidant capacity)), and radicals scavenging (ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) and DPPH (2,2-diphenyl-1-picrylhydrazyl)) assays were per-formed as previously reported (Uysal et al., 2017b). Thefindings were reported as standard compounds equivalents of EDTA or Trolox (mg EDTAE/g and mmol TE/g).
2.5. Antimicrobial assays
Gram-negative (Proteus vulgaris, Pseudomonas aeruginosa, Salmonella kentucky, Escherichia coli, Serratia marcescens, Enterobacter aerogenes) and gram-positive (Enterococcus faecium, Listeria monocytogenes ATCC 7644, Streptococcus alpha haemolyticus, Staphylococcus epidermidis, Enterococcus durans, Staphylococcus aureus ATCC 25923) bacteria were used to evaluate the antibacterial activity of PS extract. The 12 -h cul-tures of microorganisms were prepared and the suspensions were ad-justed to 0.5 McFarland standard turbidity. Serial two-fold dilutions of PS extract in a concentration range from 31.25 to 1000μg/mL were prepared and mixed with 10μL of bacterial culture in nutrient broth. The well containing nutrient broth and bacterial culture was used as positive control, whereas the well containing only nutrient broth was used as negative control. The mixtures were then incubated at 37 °C for 24 h and the absorbance at 600 nm was measured using the Multiskan Go (Thermo Scientific, USA). The minimum inhibitory concentration (MIC) values were determined as the lowest concentration that inhibit the growth of microorganisms. The minimum bactericidal concentra-tion (MBC) values were then determined by inoculating samples from the wells where microorganism growth is inhibited.
2.6. DNA protection assay
DNA protective activity of PS was analyzed as described previously (Uysal et al., 2018). Briefly, the reaction mixture consists 300 ng of pUC19 plasmid DNA, 5μL of Fenton’s reagent (80 mM FeCl3, 30 mM H2O2and 50 mM ascorbic acid), 5μL of PS extract (5–10 mg/mL) and thefinal volume was completed to 20 μL with distilled water. The ne-gative control (A) contains only the pUC19 plasmid, whereas the po-sitive control (B) consists of Fenton’s reagent and pUC19 plasmid DNA. The reaction mixtures were incubated at 37 °C for 30 min and then run on 0.8% agarose gel. The bands were quantified using Vision-Capt software.
2.7. Cytotoxicity assay 2.7.1. Cell culture
MDA-MB-231 cell line was kindly provided by the Department of Molecular Biology and Genetics, Boğaziçi University. MDA-MB-231 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS), 100 units/mL of penicillin, 100μg/mL of streptomycin, 1% non-essential amino acid (NEAA), 0.01 mg/mL human insulin and kept at 37 °C in a humidified 5% CO2 chamber.
2.7.2. MTT analysis
The cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MDA-MB-231 cells were seeded in 96-well plates at a density of 104cells/well and allowed to grow for 24 h. Serial ten-fold dilutions of PS extract were prepared in a concentration range from 0.1 to 1000μg/mL. The cells were incubated with PS extract for 24 and 48 h. Following the incubation, the culture medium was refreshed with DMEM containing 0.5 mg/mL MTT, 0.5% FBS, and incubated for 4 h at 37 °C. The purple formazan was dissolved with 3% SDS and 40 mM HCl/isopropanol and the absorbance at 570 nm was measured with a plate reader (Multiskan Go, Thermo Scientific, USA). GraphPad Prism 7 software was used to calculate the half maximal inhibitory concentration (IC50).
2.7.3. Gene expression analysis
Based on IC50concentration (118.8μg/mL), PS treatment was per-formed for 48 h at 37 °C. Total RNA was isolated using GeneJET RNA Purification Kit (Thermo Scientific). RNA quality was confirmed on 2% agarose gel after DNase treatment for 30 min at 37 °C. Reverse tran-scription of 2μg total RNA was performed with RevertAid First Strand cDNA synthesis kit (Thermo Scientific) according to manufacturer’s instructions. qPCR was performed to evaluate gene expression profiles of Bax, Bcl2, Bak1 and BIRC5. GAPDH was used for normalization and the data was analyzed with ΔΔCt method. qPCR reaction mixtures containing 10μL of SYBR Green (BioRad, USA), 1 pmol/μL of primers (Table 1),1μL of cDNA and 7 μL of dH2O were prepared and analyzed in Rotor Gene-Q Thermal Cycler (Qiagen, Germany).
2.8. Wound healing assay
MDA-MB-231 cells were seeded at a density of 25 × 104cells/well in a 6-well plate and grown until confluency. The next day, the monolayer was wounded with a 200μL pipette tip and rinsed with PBS. The cells were treated with PS for 48 h and the wound was photo-graphed in the following 24 and 48 h using an inverted microscope (Leica). Wound healing was measured by calculating the reduction in the width of the wound.
2.9. In silico evaluation 2.9.1. Enzymes preparation
In the extract of PS, have been found significant amount of caf-feoylquinic acid derivatives (6, 18a, 18b), ferulic acid derivative (9), and couramaloyl acid derivative (18). Also two other polyphenols have been found oleuropeine (21) and isoramnetin glucoside (14). The bio-logical profile of the extracts, resulted to be very active with an in-hibitory activity toward tyrosinase and in less extent towards α-glu-cosidase. In order to study the possible interaction and binding mode of the most abundant substances found in the extracts, we conducted docking experiments on the crystal structure of tyrosinase (pdb: 2Y9X) (Ismaya et al., 2011) andα-glucosidase (pdb: 3TOP) (Ren et al., 2011). The two enzyme structures have been downloaded from the PDB da-tabase and prepared as previously reported in our papers ( Llorent-Martínez et al., 2018;Yerlikaya et al., 2017).
At the beginning of the computational study, the enzyme structures need to be prepared and polished from non-catalytic water molecules
and other co-crystallized molecules present in the crystallization ma-trix. The enzymes were then submitted to a series of preparative pro-cedure embedded in the Protein Preparation Wizard (Sastry et al., 2013) of the molecular modeling suite Maestro suite 10.2 (Schrödinger, 2015). The pH was fixed pH 7.4 using PropKa module, and a mini-mization of the hydrogens only was carried out. In the case of tyr-osinase, there are present two copper ions (Cu400 and Cu401) which are part of the catalytic pocked, the metal states were generated auto-matically by Protein Preparation Wizard. After the preparation both crystallographic ligands were separated from the enzymes and used as reference for the grid docking building.
2.9.2. Ligand preparation
3-Caffeoylquinic acid (3), 5-feruloxylquinic acid (9), 3,5-di-caffeoylquinic acid (18a), 3,4 di3,5-di-caffeoylquinic acid (18b), 3-O-iso-rhamnetin-O-hexoside (14), Oleuropein (21) have been found to be the most representative compounds present in the PS extract, thus they have been selected to conduct the docking experiments (Fig. 1). The structures were manually depicted by ChemBioDraw, saved in sdf. format and loaded in Maestro, for preparation. The ligands were pre-pared by the LigPrep tool embedded in Maestro 10.2, neutralized at pH 7.4 by epiK (Greenwood et al., 2010) and minimized by OPLS3 (Harder et al., 2015) forcefield. After the preparation the molecules were saved as mol2 files and used for the docking experiments without further modification (Mollica et al., 2019).
2.9.3. Molecular docking
Considering our previous study in which we have validated the use of the docking software GOLD 6.0 to be able to perform docking on tyrosinase andα-glucosidase, we used this software also for this work. Dockings of the selected polyphenols were performed on tyrosinase and α-glucosidase. Gold 6.0 was employed for the docking calculations by using the“Gold Score” scoring function (Verdonk et al., 2003) for all the ligands (Mocan et al., 2016a;Uysal et al., 2017a) being the most reliable and powerful scoring function present in the software (Table 2). The binding pocket was determined automatically by centering the grid on the crystallographic inhibitor, extended in a radius of 10 Angstroms from the center. (Llorent-Martínez et al., 2017; Mocan et al., 2016b; Picot et al., 2017;Zengin et al., 2017).
2.10. Statistical analysis
The antimicrobial activity, DNA protection and cytotoxic analyses were performed three times. The results were expressed as mean value and standard deviation. Statistical analyses between the control and treatment groups were analyzed by using one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference post-hoc test (α = 0.05). All the analysis was performed by Minitab v17 soft-ware.
3. Results and discussion 3.1. Phytochemical profile
Secondary metabolites, ubiquitously present in plants, are known to possess numerous interesting biological activities. While assessing the
Table 1
Primer sequences used for qPCR analysis.
Gene Forward primer sequence (5′–3′) Reverse primer sequence (5′–3′)
GAPDH GGAAGGTGAAGGTCGGAGTC AACATGTAAACCATGTAGTTGAGGT
Bax CCCGAGAGGTCTTTTTCCGAG CCAGCCCATGATGGTTCTGAT
Bcl-2 GGTGGGGTCATGTGTGTGG CGGTTCAGGTACTCAGTCATCC
Bak1 ATGGTCACCTTACCTCTGCAA TCATAGCGTCGGTTGATGTCG
biological activities of plant extracts, determining the phytochemical composition is a key step. In the present study, quantitative determi-nations of the phenolic and flavonoid contents were performed. As presented inTable 3, the total phenolic content of PS methanolic leaves extract was 46.04 mg GAE/g extract, while the totalflavonoid content was 17.15 mg RE/g extract. In addition to spectrophotometric de-terminations, the extract was also analyzed using advanced chromato-graphic technique to determine the quantities and type of specific phytochemicals compounds. To our best knowledge, this is the first report which indicates phytochemical profile of PS.
The characterization of the phytochemicals was carried out by
HPLC-ESI-MSn using the negative ion mode. As can be seen from Table 4, 26 compounds were identified from PS methanolic leaves ex-tract. Most of the compounds present in the methanolic extract of PS leaves were phenolic acids, including caffeoylquinic acids, di-caffeoylquinic acids, and coumaroyilquinic acids. Compounds 2, 3, and 6 exhibited [M−H]−at m/z 353 and base peak at m/z 191, corre-sponded to caffeoylquinic acids. Similarly, compounds 16, 18, and 20 were identified as dicaffeylquinic acids based on the 515/353 frag-mentation. Compounds 16 and 20 corresponded to 4-acyl di-caffeoylquinic acids due to the MS3base peak at m/z 173, whereas18 was not substituted at position 4 (Clifford et al., 2005). Compounds5, 7, 8, and 10 (dimer) were identified as coumaroylquinic acid isomers due to the 337/191 fragmentation (Clifford et al., 2003). Similarly, compound25 was also characterized as coumaroylquinic acid due to the 337/173 fragmentation (Clifford et al., 2003). Compound11 pre-sented [M−H]−at m/z 501, and fragment ions at m/z 337 and 191, was tentatively characterized as a coumaroylquinic acid derivative. Compounds9 and 23 were tentatively characterized as 5-feruloylquinic acid (dimer) and caffeoylferuloylquinic acid isomer, respectively (Clifford et al., 2003). Compound12, [M−H]−at m/z 327, exhibited MS3fragment ions at m/z 163 and 119, was tentatively characterized as a coumaric acid derivative.
Several isorhamnetin glycosides were characterized from PS me-thanolic leaves extract. The aglycone isorhamnetin (m/z 315 and main fragment ion at m/z 300) was identified in the compounds. Compounds 13 and 24, with [M−H]−at m/z 639, suffered the neutral loss of 324 Da (hexoside + hexoside), corresponding to isorhamnetin-O-di-hexoside (Parejo et al., 2004). Compound15 which suffered the neutral loss of 294 Da (pentoside + hexoside) was characterized as iso-rhamnetin-O-pentoside-hexoside. Compounds 17 and 26 were iso-rhamnetin-O-rutinoside isomers due to the neutral loss of 308 Da to yield isorhamnetin. Compound19 was isorhamnetin-O-hexoside (loss of 162 Da). Finally, compound22, with [M−H]−at m/z 519, displayed a neutral loss of 204 Da, which was in agreement with isorhamnetin-O-acetyl-hexoside (Carazzone et al., 2013). Compound14, with [M−H]− at m/z 463, lost 162 Da to yield quercetin at m/z 301 (typical fragment ions at m/z 179 and 151), was identified as quercetin-O-hexoside.
Compound1 was identified as citric acid due to its deprotonated molecular ion at m/z 191, base peak at m/z 111, and secondary
Fig. 1. Molecular structures of the most representative compounds found in the herbal extracts.
Table 2
Dockingfitness values of the best poses scored by GoldScore function.
Compound α-glucosidase Tyrosinase
18a (3,5 isomer) 77.74 63.65 18b (3,4 isomer) 84.66 60.16 8 61.40 54.91 9 64.24 57.10 6 67.55 58.70 19 68.49 56.55 21 71.16 64.73 Table 3
Total phenolic,flavonoid content and antioxidant activity of Pittosporum senacia methanolic leaves extract.
Parameters Results
Total phenolic (mg GAE/g extract) 46.04 ± 0.93*
Totalflavonoid (mg RE/g extract) 17.15 ± 0.26
DPPH (mmol TE/g sample) 0.35 ± 0.03
ABTS (mmol TE/g sample) 0.70 ± 0.05
CUPRAC (mmol TE/g sample) 1.17 ± .05
FRAP (mmol TE/g sample) 0.65 ± 0.04
Phosphomolybdenum (mmol TE/g sample) 1.47 ± 0.05
Metal chelating (mg EDTAE/g sample) 22.65 ± 1.46
* Values expressed are means ± S.D. of three parallel measurements. GAE: Gallic acid equivalent; RE: Rutin equivalent; TE: Trolox equivalent; EDTAE: EDTA equivalent.
fragment ion at m/z 173 (Mena et al., 2012; Rjeibi et al., 2017). Compound4, [M−H]−at m/z 401, suffered the neutral loss of 132 Da (pentoside) to yield the aglycone at m/z 269. Although it seems a fla-vonoid-O-pentoside, the nature of the aglycone could not be elucidated. Compound21 was identified by comparison with an analytical standard as the glycosilated secoiridoid oleuropein. High concentration of 3-O-caffeoylquinic acid (14.0 mg/g DE) was detected.
The most abundant compounds identified in PS methanolic leaves extract were quantified by HPLC-UV, using 3-O-caffeoylquinic acid, quercetin, and oleuropein as analytical standards. The signals from phenolic acids,flavonoids, and oleuropein were recorded at 320 nm, 350 nm, and 280 nm, respectively. The results are summarized in Table 5. It was noted that the most abundant compounds were phenolic acids (chlorogenic acids), which accounted for 79.2% of the TIPC (total individual phenolic content), followed byflavonoids (17.2%). Among phenolic acids, the most abundant compounds were 3-O-caffeoylquinic acid, followed by coumaroylquinic acid, and 5-feruloylquinic acid. On the other hand, isorhamnetin glycosides accounted for 96% of the de-tected flavonoids. Finally, oleuropein represented 3.6% of the calcu-lated TIPC. The concentration of phenolics (31.2 mg/g DE) was higher compared flavonoids (6.8 mg/g DE), this finding is in line with spec-trophotometric determinations.
3.2. Antioxidant activity
Substantial amount of scientific reports has highlighted the dele-terious effects of reactive oxygen species and free radicals on the human body. Indeed, oxidative stress, caused by an excess of reactive oxygen species and free radicals in the body, has been associated to the de-velopment and exacerbation of a variety of human diseases (Carocho et al., 2018;Neha et al., 2019). In order to provide a comprehensive prediction of the antioxidant capacity of PS methanolic leaves extract, different antioxidant assays were used (Table 3). The radical scavenging properties of the extract was assessed using the DPPH and ABTS methods. The FRAP and CUPRAC assays were employed to evaluate ability of the extract to reduce ferric to ferrous and cupric to cuprous, respectively. The ability of the extract to reduce molybdenum (VI) to molybdenum (V) was also investigated. It was observed that PS me-thanolic leaves extract showed radical scavenging (0.35 and 0.70 mmol TE/g extract, for DPPH and ABTS assays, respectively) and reducing properties (1.17, 0.65, and 1.47 for CUPRAC, FRAP, and phosphomo-lybdenum assays, respectively). The acetone extracts of Pittosporum viridiflorum leaves and bark were also reported to possess radical scavenging and reducing activities (Otang et al., 2012). The ability of the extract to chelate metals, such as iron, was also determined. Iron is
Table 4
Characterization of the compounds from Pittosporum senacia methanolic leaves extract.
No. tR
(min)
[M−H]−
m/z
m/z (% base peak) Assigned identification
1 1.8 191 MS2[191]: 173 (38), 111 (100) Citric acid* 2 2.1 353 MS2[353]: 191 (100), 179 (8), 173 (6), 135 (1) MS3[353→191]: 191 (100), 173 (50), 111 (45) Caffeoylquinic acid 3 8.8 353 MS2[353]: 191 (100), 179 (5), 173 (4), 135 (1) 3-O-caffeoylquinic acid* 4 10.1 401 MS2[401]: 269 (100), 161 (64) Unknown 5 10.4 337 MS2[337]: 191 (100), 173 (17) Coumaroylquinic acid 6 11.1 353 MS2[353]: 191 (100) MS3[353→191]: 173 (100) Caffeoylquinic acid 7 12.4 337 MS2[337]: 191 (100), 173 (49), 163 (19) MS3[337→191]: 127 (100) Coumaroylquinic acid 8 13.0 337 MS2[337]: 191 (100), 163 (9) Coumaroylquinic acid 9 14.5 735 MS2[735]: 367 (100) MS3[735→367]: 191 (100)
5-Feruloylquinic acid (dimer)
10 15.0 675 MS2[675]: 337 (100), 191 (8)
MS3[675→337]: 191 (100)
Coumaroylquinic acid (dimer)
11 15.7 501 MS2[501]: 457 (67), 337 (20), 327 (9), 191 (100), 173 (11) Coumaroylquinic acid derivative
12 19.3 327 MS2[327]: 283 (100), 265 (63), 237 (22), 163 (11)
MS3[327→283]: 239 (35), 237 (42), 163 (100), 119 (31)
Coumaric acid derivative
13 20.5 639 MS2[639]: 315 (100), 300 (15) MS3[639→315]: 300 (100), 273 (15), 255 (9), 151 (12) Isorhamnetin-O-dihexoside 14 20.9 463 MS2[463]: 301 (100) MS3[463→301]: 179 (100), 151 (76) Quercetin-O-hexoside 15 21.6 609 MS2[609]: 315 (100), 300 (11) Isorhamnetin-O-pentoside-hexoside 16 22.7 515 MS2[515]: 353 (100), 179 (18), 173 (27) MS3[515→353]: 191 (22), 179 (40), 173 (100), 135 (5)
4-Acyl dicaffeoylquinic acid
17 23.5 623 MS2[623]: 315 (100), 300 (20), 271 (11), 255 (8) MS3[623→315]: 300 (100), 271 (10) Isorhamnetin-O-rutinoside 18 24.2 515 MS2[515]: 353 (100), 191 (8), 179 (7) MS3[515→353]: 191 (100), 179 (93), 135 (18) Dicaffeoylquinic acid 19 24.9 477 MS2[477]: 315 (64), 314 (100), 285 (19), 271 (10) MS3[477→315]: 300 (46), 285 (67), 271 (100) Isorhamnetin-O-hexoside 20 26.1 515 MS2[515]: 353 (100), 203 (19), 179 (8), 173 (13) MS3[515→353]: 191 (18), 179 (42), 173 (100), 135 (15)
4-Acyl dicaffeoylquinic acid
21 27.2 539 MS2[539]: 377 (100), 345 (11), 307 (66), 275 (63) MS3[539→377]: 345 (13), 307 (100), 275 (89) Oleuropein* 22 28.0 519 MS2[519]: 315 (100), 300 (16), 271 (2) Isorhamnetin-O-acetyl-hexoside 23 31.6 529 MS2[529]: 367 (19), 353 (100), 191 (19), 179 (17), 173 (21) MS3[529→353]: 191 (83), 179 (61), 173 (100) Caffeoylferuloylquinic acid 24 33.2 639 MS2[639]: 315 (100) MS3[639→315]: 300 (100), 255 (3), 151 (12) Isorhamnetin-O-dihexoside 25 36.6 483 MS2[483]: 337 (100), 319 (12), 173 (26) MS3[483→337]: 173 (100) 4-p-Coumaroylquinic acid 26 37.5 623 MS2[623]: 315 (100), 300 (25) MS3[623→315]: 300 (100), 299 (19), 271 (10), 151 (4) Isorhamnetin-O-rutinoside
an important component of many proteins and enzymes involved in physiological reactions but is also related to the catalytic decomposition of hydrogen peroxide, leading to the formation of reactive oxygen species (Jomova and Valko, 2011). Since iron plays a pivotal role in well-being and illness, controlling its level is crucial. Interestingly, PS methanolic leaves extract (22.65 mg EDTAE/g extract) showed potent iron chelating properties.
3.3. Enzyme inhibitory activity and molecular docking
The ability of PS methanol leaves extract to inhibit enzymatic ac-tivity was elucidated. Data presented clearly shows that the extract inhibited acetylcholinesterase, butyrylcholinesterase, tyrosinase, α-amylase, and α-glucosidase. It is worth mentioning that the studied extract showed high inhibitory activity against tyrosinase (65.40 mg KAE/g sample) and α-glucosidase (32.18 mmol ACAE/g sample) (Table 6). Tyrosinase andα-glucosidase inhibitors are considered as key pharmaceutical targets for the management of epidermal hy-perpigmentation problems and diabetes, respectively. Additionally, undesirable effects of current medications have fueled the need for novel, effective, and safe agents. In silico molecular docking was used to provide an insight of the possible molecular interactions of compounds
identified in PS methanol leaves extract with α-glucosidase and tyr-osinase. FromTable 2, compounds6, 8, 9, 18a, 18b, 19, and 21 had scoring fitness values greater than 50, meaning that the poses were stable and reliable. The best scoring was obtained with compound18b (3,4-dicafeoylquinic acid isomer) forα-glucosidase and compound 21 (oleuropein) for tyrosinase (Fig. 2).
Compound21 had a molecular size compatible with the enzymatic pocket of tyrosinase. The best pose revealed that oleuropein was able to establish several interactions with the amino acids surrounding the enzymatic cavity. Oleuropein interacted with Va283, Cys83, Asn81, and His85 by hydrogen bond and π−π interaction. Compound 18b strongly bound toα-glucosidase, interacting with the catalytic pocket. Indeed, compound18b was able to establish several hydrogen bonds with Asp 1279, Asp 1157, and Arg1510 and aπ−π interaction with Trp1369. Evidence from the literature supported that oleuropein in-hibited melanogenesis (Yang et al., 2016) and 3,4-Dicaffeoylquinic acid (EC50128μm), and 4,5 dicaffeoylquinic acid (EC50229μm) were found to inhibit both tyrosinase andα-glucosidase activity (Iwai et al., 2004). 3.4. Antimicrobial activity
Plant extracts have attracted substantial scientific interest as anti-microbials (Seow et al., 2014). A number of scientific studies have appraised the antimicrobials potential of plant extracts. In this study, the antimicrobial activity of PS methanolic extract was assessed against 12 Gram-negative and Gram-positive bacterial strains. For each bac-teria strains, MIC and MBC values were determined. The lowest MIC and MBC (31,25μg/ml) values were observed against Enterococcus faecium and Listeria monocytogenes (Table 7). It was noted that PS me-thanolic extract was more effective against Gram-positive bacteria compared to Gram-negative strains. Oleuropein, identified in the stu-died extract, was previously reported to inhibit growth of Gram-nega-tive and Gram-posiGram-nega-tive bacteria (Bisignano et al., 1999;Fleming et al., 1973). Oleuropein exhibited antimicrobial action by damaging the bacterial membrane and/or disrupting cell peptidoglycans (Omar, 2010). Although the interaction between oleuropein and membrane lipids have been examined in different studies (Caturla et al., 2005), the exact antibacterial mechanism of oleuropein has not been entirely un-derstood.
3.5. DNA protection
The DNA binding and protection capacity of PS methanolic extract was determined using the hydroxyl radical damage test involving Fenton Reagent's.Fig. 3 indicated DNA protection ability of PS me-thanolic extract at 5 mg/mL and 10 mg/mL. Compared to control, both concentrations had a significant protective eff ;ect against DNA damage. However, there was no significant difference between 5 mg/mL and 10 mg/mL. It can be deduced that PS methanolic extract possessed DNA protection property. DNA protection activity plays a crucial role for the determination of oxidative damage of DNA. Hydroxyl radicals can bind to DNA and cause modifications such as breakage on strands and base changes on DNA strand which can subsequently initiate cancer devel-opment. PS methanolic extract contained high amount of flavonoids especially derivatives of quercetin and isorhamnetin. Previous studies indicated that quercetin had an ability to protect nuclear DNA and mitochondrial DNA from oxidative damage (Potenza et al., 2008). In addition, quercetin was found to interact with DNA, causing induction of apoptosis in both, cancer cell lines and tumor tissues by activating intrinsic pathways (Srivastava et al., 2016). This also accords with our earlier observations, which showed that plant extract with high quer-cetin content also showed DNA protection activity. In a previous study, it was shown that high amount of quercetin in the water extract of Ononis natrix caused an increase in DNA protection activity (Yerlikaya et al., 2017). Likewise, the correlation between quercetin and initiation of apoptosis due to DNA binding ability was indicated in this study,
Table 5
Quantification of the main compounds in Pittosporum senacia methanolic leaves extract. Nº Assigned identification mg/g DE Phenolic acids 3 3-O-caffeoylquinic acid 14.0 ± 0.5 5 Coumaroylquinic acid 0.151 ± 0.007 6 Caffeoylquinic acid 0.22 ± 0.01 7 Coumaroylquinic acid 0.72 ± 0.02 8 Coumaroylquinic acid 4.2 ± 0.2 9 5-Feruloylquinic acid 2.7 ± 0.1 10 Coumaroylquinic acid 0.92 ± 0.05
16 4-Acyl dicaffeoylquinic acid 0.58 ± 0.04
18 Dicaffeoylquinic acid 3.2 ± 0.2
20 4-Acyl dicaffeoylquinic acid 3.8 ± 0.2
23 Caffeoylferuloylquinic acid 0.44 ± 0.02 25 4-p-Coumaroylquinic acid 0.29 ± 0.02 Total 31.2 ± 0.6 Flavonoids 14 Quercetin-O-hexoside 0.28 ± 0.01 15 Isorhamnetin-O-pentoside-hexoside 0.40 ± 0.03 17 Isorhamnetin-O-rutinoside 0.64 ± 0.03 19 Isorhamnetin-O-hexoside 2.4 ± 0.1 22 Isorhamnetin-O-acetyl-hexoside 1.15 ± 0.06 24 Isorhamnetin-O-dihexoside 1.10 ± 0.05 26 Isorhamnetin-O-rutinoside 0.79 ± 0.03 Total 6.8 ± 0.2 Others 21 Oleuropein 1.42 ± 0.04 TIPC 39.4 ± 0.6
TIPC = Total individual phenolic content; defined as the sum of all the quan-tified compounds.
Table 6
Enzyme inhibitory activity of Pittosporum senacia methanolic leaves extract.
Parameters Results
AChE Inhibition (mg GALAE/g sample) 1.87 ± 0.04*
BChE Inhibition (mg GALAE/g sample) 1.88 ± 0.15
Tyrosinase inhibition (mg KAE/g sample) 65.40 ± 3.44
α-Amylase inhibition (mmol ACAE/g sample) 0.48 ± 0.03
α-Glucosidase inhibition (mmol ACAE/g sample) 32.18 ± 0.46
* Values expressed are means ± S.D. of three parallel measurements. GALAE: Galatamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent.
Ononis natrix water extract caused induction of apoptosis for prostate cancer cell line, PC3. Consistent with the literature, this research also found that PS methanolic extract had a great DNA binding capability which provides initiation of apoptosis in human breast cancer cell line, MDA-MB-231. It can be suggested that quercetin, identified in PS me-thanolic extract, could be responsible for the observed DNA protection and apoptosis induction.
3.6. Cytotoxic activity
The cytotoxic property of PS methanolic extract on the survival of human breast cancer cell line, MDA-MB-231, was evaluated using MTT cell viability test. MDA-MB-231 cells were exposed to different doses of extracts (0.1, 1, 10, 100, and 1000μg/mL) for 24 and 48 h. Cells treated with 0.5% DMSO were used as control. After application of PS metha-nolic extract on MDA-MB-231 cells, cell morphology was firstly ex-amined under inverted microscope (Fig. 4a). According to results, the effect of PS methanolic extract on the decrease in cell number and
change in cellular morphology was observed. In order to show the cy-totoxic effect of PS methanolic extract, cell viability assay known as MTT was also performed on MDA-MB-231cells. There was no sig-nificant reduction in cell number after treatment with 0.1, 1 and 10 μg/ mL extract for 24 and 48 h. However, PS methanolic extract at 100 and 1000μg/mL significantly decreased the survival rate of MDA-MB-231 cells for both time periods (Fig. 4b). In addition, IC50value for 48 h of PS treatment was calculated to find out optimum concentration (Fig. 4c). This IC50value (118.8μg/mL) was used for qPCR and cell migration tests. Because the utilization of low concentration doses with time-dependent manner caused an increase in cytotoxic eff ;ect, time and dose dependent anti-proliferative effect of PS methanolic extract on MDA-MB-231 cells were observed.
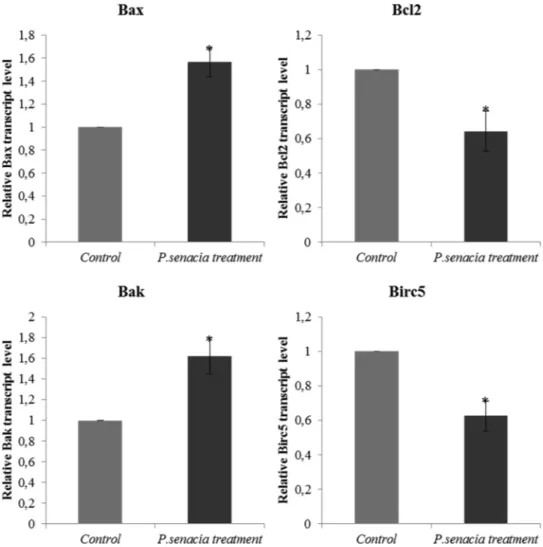
We also investigated into the mechanism behind the cytotoxic effect of PS methanolic extract on epithelial adenocarcinoma breast cancer cell line, MDA-MB-231. For this purpose, we determined the expression profile of apoptosis-associated genes including apoptosis inhibitors (e.g., Bcl-2 and Birc5) and apoptosis promoters (e.g., Bax and Bak). Bax to Bcl-2 and Bak to Birc5 ratios were compared after the application of PS methanolic extract at 118.8μg/mL. In both cases, gene expression of apoptosis promoter genes was significantly higher than apoptosis in-hibitor genes (Fig. 5), meaning that after plant extract treatment, gene expressions of Bax and Bak increased whereas gene expression level of Bcl-2 and Birc5 decreased compared to controls. In addition to control of apoptosis markers, wound healing assay was performed and results were presented inFig. 6. For the control group, cell migration was very active and approximately 50% of healing was achieved after 24 h. The scrapped region was completely closed for control samples after 48 h. However, inhibition of cell migration was noted upon treatment with PS methanolic extract for 24 and 48 h.
Over the past decades there has been a steep increase in cancer prevalence, incidence, and mortality, making it the leading cause of death worldwide (Segun et al., 2018). Medicinal plants are considered as promising sources of anticancer bioactive agents. The quest for an-ticancer agents from plants started in 1950 with the discovery of Vinca alkaloids (Benarba and Pandiella, 2018). Plant bioactive secondary
Fig. 2. Best ranked pose of 3,4-dicaffeoylquinic acid (18b) (A–B) on α-glucosidase and oleuropein (21) docked to tyrosinase (C–D).
Table 7
The MIC and MBC values of Pittosporum senacia extract against the micro-organisms tested. Microorganisms MIC MBC Proteus vulgaris 250 1000 Pseudomonas aeruginosa 250 1000 Salmonella kentucky 250 1000 Escherichia coli 250 1000 Serratia marcescens 250 1000 Enterobacter aerogenes 250 1000 Enterococcus faecium 31,25 31,25 Listeria monocytogenes 31,25 31,25
Streptococcus alpha haemolyticus 250 500
Staphylococcus epidermidis 250 1000
Enterococcus durans 250 1000
Staphylococcus aureus 250 1000
MBC: minimal bactericidal concentration (μg/ml); MIC: minimal inhibition concentration (μg/ml).
metabolites have shown their ability to inhibit cancer cell growth by different mechanisms, such as, regulation of cell apoptosis, autophagy, immune system, and inhibition of angiogenesis (Baloglu et al., 2019; Mahomoodally et al., 2019;Marwan Almosnid et al., 2018). There is a paucity of research geared towards on the evaluation of Pittosporum species as anticancer agents. This study evaluated the cytotoxic prop-erty of PS methanolic extract on MDA-MB-231 cells for thefirst time. The cytotoxic property of chlorogenic acid (3-O-caffeoylquinic acid), present in PS methanolic extract in significant amount, was also de-termined. It was found that the anticancer property of the PS
methanolic extract was similar to chlorogenic acid. In a recent study, it was reported that chlorogenic acid had an ability for regulation of gene expression associated with apoptosis and self-renewal-related in cancer cells (Kazuo Yamagata, 2018). They indicated that chlorogenic acid diminished cell proliferation in lung cancer cell line A549 based on MTT results. This decrease was demonstrated by determining Bax to Bcl-2 ratio which showed a reduction in Bcl-2 expression and an in-crease in Bax expression. These results match those observed in our study. It was thus suggested that chlorogenic acid might be responsible for the induction of apoptosis via the stimulation of some
apoptosis-Fig. 3. DNA protection assay. A, pUC19 DNA (0.3μg); B, pUC19 DNA (0.3 μg) + Fenton’s reagent; C, pUC19 DNA (0.3 μg) + Pittosporum senacia extract (5 mg/ml) + Fenton’s reagent; D, pUC19 DNA (0.3 μg) + Pittosporum senacia extract (10 mg/ml) + Fenton’s reagent. Mean values of 3 independent experiments were graphically represented; *, p < 0.05.
Fig. 4. a. Cellular morphology of MDA-MB-231 cells after Pittosporum senacia treatment for 24 h and 48 h. Control cells were treated with 0.1% DMSO. b. MTT analysis results. MDA-MB-231 cells were treated with Pittosporum senacia extracts at increasing concentrations for 24 h and 48 h.c. IC50value for 48 h of P. senacia
associated genes. Chlorogenic acid has been previously reported to in-hibit cell proliferation through apoptosis mechanism in a human acute promyelocytic leukemia cell line (Liu et al., 2013). Recently, it was reported that chlorogenic acid and its microbial metabolites showed anti-proliferative effects in human colon cancer cells Caco-2 via reg-ulation of apoptosis (Sadeghi Ekbatan et al., 2018). It might be argued that chlorogenic acid present in PS methanolic extract might be related to cell growth inhibition of breast cancer line via similar apoptotic pathways.
4. Conclusion
The present study appraised the antioxidant and enzyme inhibitory properties of PS methanolic leaves extract, for thefirst time. Data col-lected showed that the extract was a potent inhibitor of tyrosinase and α-glucosidase, enzymes targeted for the management of epidermal hyperpigmentation problems and diabetes, respectively. Phytochemical profiling revealed that PS methanolic leaves extract was rich in 3-O-caffeoylquinic acid. The in silico studies reported that compound 21 and 18b, showed strong interactions with tyrosinase and α-glucosidase, respectively. The antimicrobial activity of PS methanolic extract ex-amined using 12 Gram-negative and Gram-positive bacterial species and found Enterococcus faecium and Listeria monocytogenes bacteria strains were the most affected ones after application of plant extracts. In addition, PS methanolic extract had an ability to interact with DNA and showed high DNA protective activity. PS methanolic extract possessed
also anti-proliferative effect on MDA-MB-231 cells. According to MTT results, IC50 value was calculated and 118.8μg/ml plant extract dis-played time and dose dependent anti-cancer activity on MDA-MB-231 cells. Expression profiles of apoptosis inhibitor genes such as Bcl-2 and Birc5 and apoptosis promoter genes such as Bax and Bak were con-trolled after the treatment of plant extract. Gene expressions of apop-tosis promoter genes augmented but gene expression level of apopapop-tosis inhibitor genes diminished compared to controls. Inhibition of cell migration was also observed after the treatment of PS methanolic ex-tract on MDA-MB-231 cells. Due to the high amount of chlorogenic acid, quercetin and oleuropein in methanolic PS extract, the growth of MDA-MB-231 cells were probably inhibited by apoptosis mechanism. Scientific evidence gathered in the present study support the need for further study which might lead to the development of new therapeutic entities for the management of the above-mentioned ailments.
Author contribution
MFM, CP, MH: Plant providing and data analysis. AU, MC: Cell assays.
AM, AS: Molecular docking. EJL: HPLC analysis.
GZ: Antioxidant and enzyme inhibitory assays.
Fig. 5. Pro-apoptotic and anti-apoptotic marker gene expressions. MDA-MB-231 cells were treated with Pittosporum senacia extract at IC50concentration for 48 h.
Control cells were treated with 0.1% DMSO. Transcript levels of the marker genes were analyzed in total RNA by qPCR. GAPDH gene was used as internal control. *, p < 0.05.
Declaration of Competing Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant fi-nancial support for this work that could have influenced its outcome. References
Andrade, S., Ramalho, M.J., Loureiro, J.A., Pereira, M.D., 2019. Natural compounds for Alzheimer’s disease therapy: a systematic review of preclinical and clinical studies. Int. J. Mol. Sci. 20, 41.
Ayaz, M., Ullah, F., Sadiq, A., Ullah, F., Ovais, M., Ahmed, J., Devkota, H.P., 2019. Synergistic interactions of phytochemicals with antimicrobial agents: potential strategy to counteract drug resistance. Chem.-Biol. Interact. 308, 294–303.
Aziz, M.M., Raza, M.A., Saleem, H., Wajid, M., Bashir, K., Ikram, M., 2014. Medicinal values of herbs and plants, importance of phytochemical evaluation and ethno-pharmacological screening: an illustrated review essay. J. Pharm. Cosmetic Sci. 2, 6–10.
Baloglu, M.C., Llorent-Martínez, E.J., Aumeeruddy, M.Z., Mahomoodally, M.F., Altunoglu, Y.C., Ustaoglu, B., Ocal, M., Gürel, S., Bene, K., Sinan, K.I., Zengin, G., 2019. Multidirectional insights on Chrysophyllum perpulchrum leaves and stem bark extracts: HPLC-ESI-MSn profiles, antioxidant, enzyme inhibitory, antimicrobial and cytotoxic properties. Ind. Crop. Prod. 134, 33–42.
Benarba, B., Pandiella, A., 2018. Colorectal cancer and medicinal plants: principle find-ings from recent studies. Biomed. Pharmacother. 107, 408–423.
Bisignano, G., Tomaino, A., Cascio, R.L., Crisafi, G., Uccella, N., Saija, A., 1999. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 51, 971–974.
Carazzone, C., Mascherpa, D., Gazzani, G., Papetti, A., 2013. Identification of phenolic constituents in red chicory salads (Cichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Food Chem. 138, 1062–1071.
Carocho, M., Morales, P., Ferreira, I.C.F.R., 2018. Antioxidants: reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 71, 107–120.
Caturla, N., Pérez-Fons, L., Estepa, A., Micol, V., 2005. Differential effects of oleuropein, a biophenol from Olea europaea, on anionic and zwiterionic phospholipid model membranes. Chem. Phys. Lipid. 137, 2–17.
Clifford, M.N., Johnston, K.L., Knight, S., Kuhnert, N., 2003. Hierarchical scheme for LC-MS n identification of chlorogenic acids. J. Agric. Food Chem. 51, 2900–2911.
Clifford, M.N., Knight, S., Kuhnert, N., 2005. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MS n. J. Agric. Food Chem. 53, 3821–3832.
Dutt, R., Garg, V., Khatri, N., Madan, A.K., 2019. Phytochemicals in anticancer drug development. Anticancer Agents Med. Chem. 19, 172–183.
Fleming, H.P., Walter Jr., W.M., Etchells, J.L., 1973. Antimicrobial properties of oleur-opein and products of its hydrolysis from green olives. Appl. Microbiol. 26, 777–782.
Greenwood, J.R., Calkins, D., Sullivan, A.P., Shelley, J.C., 2010. Towards the compre-hensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. J. Comput. Aid Mol. Des. 24, 591–604.
Harder, E., Damm, W., Maple, J., Wu, C., Reboul, M., Xiang, J.Y., Wang, L., Lupyan, D., Dahlgren, M.K., Knight, J.L., 2015. OPLS3: a forcefield providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput. 12, 281–296.
Ismaya, W.T., Rozeboom, H.J., Weijn, A., Mes, J.J., Fusetti, F., Wichers, H.J., Dijkstra, B.W., 2011. Crystal structure of Agaricus bisporus mushroom tyrosinase: identity of the tetramer subunits and interaction with tropolone. Biochemistry 50, 5477–5486.
Iwai, K., Kishimoto, N., Kakino, Y., Mochida, K., Fujita, T., 2004. In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans. J. Agric. Food Chem. 52, 4893–4898.
Jomova, K., Valko, M., 2011. Importance of iron chelation in free radical-induced oxi-dative stress and human disease. Curr. Pharm. Des. 17, 3460–3473.
Kazuo Yamagata, Y.I., Onodera, Daiki, Tagami, Motoki, 2018. Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in A549 human lung cancer cells. Mol. Cell. Biochem. 441, 9–19.
Linnek, J., Mitaine‐Offer, A.C., Paululat, T., Lacaille‐Dubois, M.A., 2012. Two new tri-terpenoid saponins from Pittosporum senacia Putterlick (Pittosporaceae). Magn. Reson. Chem. 50, 798–802.
Liu, Y., Z.C, Qiu, C., Lu, X., Wang, Y., 2013. Chlorogenic acid induced apoptosis and inhibition of proliferation in human acute promyelocytic leukemia HL‑60 cells. Mol. Med. Rep. 8, 1106–1110.
Llorent-Martínez, E.J., Zengin, G., Fernández-de Córdova, M.L., Bender, O., Atalay, A., Ceylan, R., Mollica, A., Mocan, A., Uysal, S., Guler, G.O., 2017. Traditionally used Lathyrus species: phytochemical composition, antioxidant activity, enzyme inhibitory properties, cytotoxic effects, and in silico studies of L. czeczottianus and L. nissolia. Front. Pharmacol. 8, 83.
Llorent-Martínez, E.J., Zengin, G., Lobine, D., Molina-García, L., Mollica, A., Mahomoodally, M.F., 2018. Phytochemical characterization, in vitro and in silico approaches for three Hypericum species. New J. Chem. 42, 5204–5214.
Maher, P., 2019. The potential offlavonoids for the treatment of neurodegenerative diseases. Int. J. Mol. Sci. 20, 3056.
Mahomoodally, M., Gurib‐Fakim, A., Subratty, A., 2010. Screening for alternative anti-biotics: an investigation into the antimicrobial activities of medicinal food plants of Mauritius. J. Food Sci. 75, 173–177.
Mahomoodally, M.F., Yerlikaya, S., Llorent-Martínez, E.J., Uğurlu, A., Baloglu, M.C., Altunoglu, Y.C., Mollica, A., Dardenne, K.K., Aumeeruddy, M.Z., Puchooa, D., Zengin, G., 2019. Pharmacological and polyphenolic profiles of Phyllanthus phillyreifolius var. commersonii Müll. Arg: an unexplored endemic species from Mauritius. Food Res. Int. 115, 425–438.
Marwan Almosnid, N., Zhou, X., Jiang, L., Ridings, A., Knott, D., Wang, S., Wei, F., Yuan, J., Altman, E., Gao, Y., Miao, J., 2018. Evaluation of extracts prepared from 16 plants used in Yao ethnomedicine as potential anticancer agents. J. Ethnopharmacol. 211, 224–234.
Mena, P., Calani, L., Dall’Asta, C., Galaverna, G., García-Viguera, C., Bruni, R., Crozier, A., Del Rio, D., 2012. Rapid and comprehensive evaluation of (poly) phenolic com-pounds in pomegranate (Punica granatum L.) juice by UHPLC-MSn. Molecules 17, 14821–14840.
Fig. 6. Effect of Pittosporum senacia treatment on cell migration. MDA-MB-231 cells were treated with Pittosporum senacia extract at IC50concentration for 48 h.
Control cells were treated with 0.1% DMSO. The images were obtained with inverted microscope. Relative distance in each wound was measured for quantitation of the cell migration.
Mocan, A., Zengin, G., Crişan, G., Mollica, A., 2016a. Enzymatic assays and molecular modeling studies of Schisandra chinensis lignans and phenolics from fruit and leaf extracts. J. Enzym. Inh. Med. Chem. 31, 200–210.
Mocan, A., Zengin, G., Uysal, A., Gunes, E., Mollica, A., Degirmenci, N.S., Alpsoy, L., Aktumsek, A., 2016b. Biological and chemical insights of Morina persica L.: a source of bioactive compounds with multifunctional properties. J. Funct. Food. 25, 94–109.
Mollica, A., Zengin, G., Durdagi, S., Ekhteiari Salmas, R., Macedonio, G., Stefanucci, A., Dimmito, M.P., Novellino, E., 2019. Combinatorial peptide library screening for discovery of diverseα-glucosidase inhibitors using molecular dynamics simulations and binary QSAR models. J. Biomol. Struct. Dyn. 37, 726–740.
Neha, K., Haider, M.R., Pathak, A., Yar, M.S., 2019. Medicinal prospects of antioxidants: a review. Eur. J. Med. Chem. 178, 687–704.
Omar, S.H., 2010. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 78, 133–154.
Otang, W.M., Grierson, D.S., Ndip, R.N., 2012. Phytochemical studies and antioxidant activity of two South African medicinal plants traditionally used for the management of opportunistic fungal infections in HIV/AIDS patients. BMC Complement. Altern. Med. 12, 43.
Oyenihi, A.B., Smith, C., 2019. Are polyphenol antioxidants at the root of medicinal plant anti-cancer success? J. Ethnopharmacol. 229, 54–72.
Parejo, I., Jauregui, O., Sánchez-Rabaneda, F., Viladomat, F., Bastida, J., Codina, C., 2004. Separation and characterization of phenolic compounds in fennel (Foeniculum vulgare) using liquid chromatography− negative electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 52, 3679–3687.
Picot, M.C., Zengin, G., Mollica, A., Stefanucci, A., Carradori, S., Mahomoodally, M., 2017. In vitro and in silico studies of mangiferin from Aphloia theiformis on key en-zymes linked to diabetes type 2 and associated complications. Med. Chem. 13, 633–640.
Potenza, L., Calcabrini, C., De Bellis, R., Mancini, U., Cucchiarini, L., Dachà, M., 2008. Effect of quercetin on oxidative nuclear and mitochondrial DNA damage. BioFactors 33, 33–48.
Ren, L., Qin, X., Cao, X., Wang, L., Bai, F., Bai, G., Shen, Y., 2011. Structural insight into substrate specificity of human intestinal maltase-glucoamylase. Protein Cell 2, 827–836.
Rengasamy, K.R.R., Khan, H., Gowrishankar, S., Lagoa, R.J.L., Mahomoodally, F.M., Khan, Z., Suroowan, S., Tewari, D., Zengin, G., Hassan, S.T.S., Pandian, S.K., 2019. The role offlavonoids in autoimmune diseases: therapeutic updates. Pharmacol. Ther. 194, 107–131.
Rjeibi, I., Ncib, S., Saad, A.B., Souid, S., 2017. Evaluation of nutritional values, phenolic profile, aroma compounds and biological properties of Pittosporum tobira seeds. Lipids Health Dis. 16, 206.
Sadeghi Ekbatan, S., Li, X.-Q., Ghorbani, M., Azadi, B., Kubow, S., 2018. Chlorogenic acid and its microbial metabolites exert anti-proliferative effects, S-phase cell-cycle arrest and apoptosis in human colon cancer Caco-2 cells. Int. J. Mol. Sci. 19, 723.
Saleem, H., Anjum, S.M.M., Awan, A.B., Ahmed, J., 2013. A short history of evolution of
indigenous plants and medicine system. J. Pharm. Alt. Med. 2, 1–4.
Sastry, G.M., Adzhigirey, M., Day, T., Annabhimoju, R., Sherman, W., 2013. Protein and ligand preparation: parameters, protocols, and influence on virtual screening en-richments. J Comput. Aid. Mol. Des. 27, 221–234.
Schrödinger, 2015. 2017-3: Maestro, Schrödinger. LLC, New York.
Segun, P.A., Ogbole, O.O., Ajaiyeoba, E.O., 2018. Medicinal plants used in the manage-ment of cancer among the ijebus of southwestern Nigeria. J. Herb. Med. 14, 68–75.
Seow, Y.X., Yeo, C.R., Chung, H.L., Yuk, H.-G., 2014. Plant essential oils as active anti-microbial agents. Crit. Rev. Food Sci. Nutr. 54, 625–644.
Srivastava, S., Somasagara, R.R., Hegde, M., Nishana, M., Tadi, S.K., Srivastava, M., Choudhary, B., Raghavan, S.C., 2016. Quercetin, a naturalflavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci. Rep. 6, 24049.
Suroowan, S., Pynee, K., Mahomoodally, M., 2019. A comprehensive review of ethno-pharmacologically important medicinal plant species from Mauritius. S. Afr. J. Bot. 122, 189–213.
Uysal, S., Aktumsek, A., Picot, C.M., Sahan, A., Mollica, A., Zengin, G., Mahomoodally, M.F., 2017a. A comparative in vitro and in silico study of the biological potential and chemicalfingerprints of Dorcycinum pentapyllum subsp. haussknechtii using three ex-traction procedures. New J. Chem. 41, 13952–13960.
Uysal, S., Ugurlu, A., Zengin, G., Baloglu, M.C., Altunoglu, Y.C., Mollica, A., Custodio, L., Neng, N.R., Nogueira, J.M., Mahomoodally, M.F., 2018. Novel in vitro and in silico insights of the multi-biological activities and chemical composition of Bidens tripartita L. Food Chem. Toxicol. 111, 525–536.
Uysal, S., Zengin, G., Locatelli, M., Bahadori, M.B., Mocan, A., Bellagamba, G., De Luca, E., Mollica, A., Aktumsek, A., 2017b. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical com-position. Front. Pharmacol. 8, 290.
Verdonk, M.L., Cole, J.C., Hartshorn, M.J., Murray, C.W., Taylor, R.D., 2003. Improved protein-ligand docking using GOLD. Proteins 52, 609–623.
Yang, J., Wu, S., Xu, H., Yan, Y., Ju, B., Zhu, D., Liang, X., Hu, J., 2016. Inhibitory effects of phenylethanoid glycosides on melanin synthesis in cultured human epidermal melanocytes. Int. J. Clin. Exp. Med. 9, 18019–18025.
Yerlikaya, S., Zengin, G., Mollica, A., Baloglu, M.C., Celik Altunoglu, Y., Aktumsek, A., 2017. A Multidirectional perspective for novel functional products: in vitro phar-macological activities and in silico studies on Ononis natrix subsp. hispanica. Front. Pharmacol. 8.
Zengin, G., Mollica, A., Aktumsek, A., Picot, C.M.N., Mahomoodally, M.F., 2017. In vitro and in silico insights of Cupressus sempervirens, Artemisia absinthium and Lippia tri-phylla: bridging traditional knowledge and scientific validation. Eur. J. Integr. Med. 12, 135–141.
Zengin, G., Senkardes, I., Mollica, A., Picot-Allain, C.M.N., Bulut, G., Dogan, A., Mahomoodally, M.F., 2018. New insights into the in vitro biological effects, in silico docking and chemical profile of clary sage–Salvia sclarea L. Comput. Biol. Chem. 75, 111–119.