Investigation of structural, optical and dielectrical properties
of Cu
2WS
4thin film
Murat Yıldırım1 · Faruk Özel2 · Adem Sarılmaz2 · Abdalaziz Aljabour3 · İmren Hatay Patır1
Received: 29 October 2016 / Accepted: 6 January 2017 / Published online: 20 January 2017 © Springer Science+Business Media New York 2017
1 Introduction
Ternary compound materials are of great interest because of their suitable optical and electrical properties. The prop-erties of these materials exhibit such qualities as
semi-conductor [1], plasmonic material [2], narrow band-gap
[3], high photocatalytic [4], ferromagnetism
andantifer-romagnetism [5]. Recently, sulfides which are one of the
ternary compound materials have been promising as photo-catalytic for hydrojen production because the sulfides have
been studied extensively [6]. The ternary metal
chalcoge-nide Cu2WS4 was first prepared by Pruss who used a low
temperature synthesis method [7]. Pruss has adapted and
used to make chalcogenide materials, including four other
members of the Cu2MX4 family. (M = W or Mo and X = S
or Se or S/Se). Pruss reacted a mixture of (NH4)2WS4 and
Cu(CH3CN)4BF4 in a 1:1 mixture of
N,N-dimethylforma-mide (DMF) and butyronitrile at reflux (135 °C) for 24 h.
Crossland et al. [8, 9] have reported similar reactions in
which reagents were dissolved in a mixture of DMF and butyronitrile and heated in a Teflon lined hydrothermal autoclave for a certain time between 6 and 96 h. Crossland
et al. have chosen Cu(CH3CN)4BF4 salt the same as Pruss
et al., because the CH3CN ligands are relatively labile and
easily displaced by MX4. The tungsten and molybdenum
sources were materials in which MX4 tetrahedra already
existed and these structural units were preserved during the
reaction and are linked together by the Cu+ ions to enable
extended structure. For the sulphide materials, ammonium
tetrathiotungstate/molybdate was the source of MX4 and for
the selenide materials the corresponding
tetraphenylphos-phonium salt was used instead. Jaramillo et al. [10] have
showed that sintering at a higher temperature (823 K) led to larger nanoclusters than annealing at lower temperature (673 K). Jing et al. have prepared W-based chalcogenide
Abstract Ternary I-Cu2WS4 were synthesized based on
hot-injection process and their thin films are prepared by spin coating techniques at ambient temperature. The energy dispersive analysis of X-rays of the thin films confirmed that synthesized thin film is stoichiometric. Transmittance and reflectance have been used to determine the optical,
dispersion and dielectric properties of the Cu2WS4 in the
range of 200–2400 nm. The transparency of the Cu2WS4
is 40–45% in the visible range. Optical dispersion param-eters have been calculated by using the single term Sell-meier dispersion relation and Wemple–DiDomenico sin-gle oscillator model. Several dispersion parameters were determined by the analysis of refractive index dispersion. Absorption coefficient (α), extinction coefficient (k), the
Urbach energy (EU), real and imaginary parts of dielectric
constant (ε) and surface and volume energy loss function have been calculated. The optical bandgap determined by the optical absorbance spectrum analysis showed that thin films possess a direct bandgap of 1.74 eV.
* Murat Yıldırım
muratyildirim@selcuk.edu.tr
1 Department of Biotechnology, Faculty of Science, Selcuk
University, 42030 Konya, Turkey
2 Department of Metallurgical Science and Materials
Engineering, Faculty of Engineering, Karamanoğlu Mehmetbey University, 70200 Karaman, Turkey
3 Department of Chemistry, Faculty of Science, Selcuk
Cu2WS4 by a facile hydrothermal method [11]. Their method was demonstrated to be simple, convenient and of
high yield. Cu2WS4 photocatalysts were of special
deca-hedral structure and with nearly energy gap ≈ 2.1 eV. They found that the crystallite decahedron was about 200 nm and 100–1000 nm thick and wide, respectively. The
hydro-thermal method avoids the traditional use of H2S for the
preparation of such chalcogenide, which guarantees an environmental-friendly process. According to the study of
Li et al., Cu2WS4 was synthesized by hydrothermal method
at 200 °C for 72 h [12]. Gan et al. [13] have reported the
structural parameters and band gaps of C2WS4 compound
were obtained using the Perdew-Burke-Ernzerhof (PBE)
[14], PBE +(vdW) [15] (with van der Waals (vdW)
cor-rection) and Heyd-Scuseria-Ernzerhof (HSE06) [16, 17]
functions. The band gaps of C2WS4 values have been
deter-mined by these functions are 1.43, 1.24 and 2.15
eVre-spectively. Chaki et al. [18] have synthesized the CuS thin
films on a glass substrate by chemical bath deposition and
dip coating technique. They [18] have determined that the
direct optical bandgap values from the optical absorbance spectra of chemical bath deposition and dip coating of syn-thesized CuS thin films are 2.2 and 2.5 eV, respectively.
Chaki et al. [19] have reported that the direct optical
band-gap values of undoped and Mn doped CuS nanoparticles were determined 1.6 and 1.7 eV, respectively. The obtained optical band gap value of the Mn doped CuS nanoparticles is more than the undoped CuS nanoparticles. They showed that Mn doped CuS nanoparticles were more stable than undoped CuS nanoparticles by conducting differential
ther-mal analysis (DTA) [19]. The optical constants such as the
optical band gap, refractive index and extinction coefficient
are usually dependent on the film thickness [20]. Although
the optical properties of this nanocrystalline CuS binary
system have been studied [18], the effect of adding W and
the thin film properties of ternary system has not been stud-ied in details.
For the design of optoelectronic devices, precise infor-mation of the optical dispersion and dielectric parameters is a significant factor. There are many reports on the optical constants, the dispersion parameters, the dielectric param-eters, the electric modulus, the surface energy loss function and the volume energy loss function parameter of related compounds in the available literature. However, there are not any reports on these parameters of related compounds
of Cu2WS4 in available literature. In this study, we have
reported that a detailed analysis to determine optical
prop-erties of the thin film of ternary Cu2WS4 which is a
synthe-sis with hot injection method. High-quality nanoparticles can be obtained and also composed and size distribution of the compound can be controlled by this method. The thin
film of Cu2WS4 was prepared by spin coating on
Corn-ing-1737 glass substrate. Furthermore, the transmittance
and reflectance spectra are measured in the wavelength range 200–2400 nm and the energy gap of the nanoparti-cles is also estimated.
2 Experimental
In this study, we have reported that a detailed analysis to
determine optical properties of I phase Cu2WS4 as a thin
film which is synthesized via the hot injection method. High quality nanoparticles can be obtained and also com-posed of and size distrubition of the compound can be
controlled by this method. Synthesis of Cu2WS4
nanoparti-cles was carried out by depending on previously published
procedure [21]. The Cu2WS4 nanoparticles were made by
the use of Copper(II) chloride dihydrate (CuCl2·2H2O),
tungsten(IV) chloride (WCl4), Sulfur powder and Ethanol
supplied from Sigma–Aldrich. Oleylamine 80–90% (OLA)
was purchased from Acros Organic. OleicAcid (C18H34O2)
was supplied from Fisher Scientific. Toluene was pur-chased from VWR.
Cu2WS4 nanoparticles were synthesized by
depend-ing on the hot-injection process by usdepend-ing metal salts pre-cursors as starting materials and a surfactant as a capping agent. 1 mmol Copper(II) chloride dihydrate, 0.5 mmol tungsten(IV) chloride and 10 ml OLA are mixed in a 25 ml three-neck flask and heated till 180 °C under Ar flow. When a greenish-blue solution is observed, 1 ml OLA including 2 mmol sulfur powder is added to reaction flask. Then, the temperature is increased to 300 °C and kept for 30 min. by stirring. The reaction mixture is cooled to 80 °C and 2 ml oleic acid is added to reaction flask. Toluene- ethanol (7:1)
mixture is added and centrifuged to precipitated Cu2WS4
nanoparticles. Finally, nanoparticles are washed several times with ethanol and left to dry at 70 °C for 2 h.
We have prepared a solution from Cu2WS4 nanoparticles
dispersed in toluene. The prepared solution was stirred for
30 min and spin casted with Cu2WS4 solution at 1500 rpm
spin speed for 60 s on Corning-1737 glass at room tempera-ture. Prior to coating, glass substrate was cleaned in trichlo-roethylene, methanol and acetone for 5 min each by using an ultrasonic cleaner and then cleaned with 18 MΩ cm
resistivity deionized water and dried in N2 gas. The glass
substrate was finally cleaned by ultraviolet-generated ozone (UVO) cleaner model no 42–220 (Jelight Company, USA).
The thickness measurement of the coated Cu2WS4 thin
film was performed by stylus profilometre Veeco Dektak 150.The X-ray diffraction (XRD) measurements of col-loidal nanoparticles were made in a Bruker diffractom-eter, model Advance D8 using the Cuα source with the wavelength of the 1.5406 Å. The morphology studies and chemical analyses were made with a ZeissEvo scanning electron microscope with a dispersive energy detector. The
microscopic images and interplanar spacings were ana-lysed with a JEOL JEM-2100F model transmission elec-tron microscope (TEM). Optical transmittance and reflec-tance spectra of the samples were obtained by UV-VIS-NIR photospectrometer, with a wide spectral range from 200 to 2400 nm, at room temperature on a Jasco V-670 photospectrometer.
3 Results and discussion
3.1 Characterization and structural properties of Cu2WS4 nanoparticles
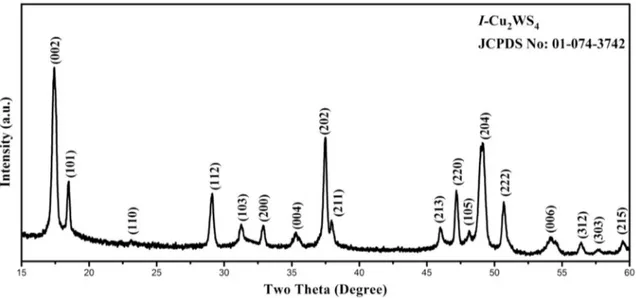
The X-ray diffraction (XRD) pattern of synthesized
Cu2WS4 nanoparticles by hot injection method is shown in
Fig. 1. All the diffraction peaks can be indexed to the body
centered tetragonal type of I-Cu2WS4 with lattice
param-eters 5.45 Å for a and b, 10.08 Å for c, which are very close to the reported data in the literature (JCPDS No: 01-074-3742). Additionally, no characteristic peaks of impurities (such as CuS and WS) were detected in the diffraction pat-tern, which showed that synthesized the nanoparticles had high purity.
As shown Fig. 2, homogeneity and composition of
Cu2WS4 nanoparticles are examined by Energy-dispersive
X-ray spectroscopy (EDS). According to obtained EDS results, the average elemental composition (%) ratio was found as 2:1, 1:4 and it was seen that these rates were close to ideal composition.Furthermore, these result showed that nanoparticles in ideal ratios can be obtained with hotinjec-tion method.
Transmission electron microscopy (TEM), high resolu-tion TEM (HR-TEM) and selected area electron diffracresolu-tion
(SAED) images of the Cu2WS4 nanoparticles are given
in Fig. 3a, b, c, respectively. As it can be clearly seen in
Fig. 3a, the nanoparticles have cubic and rectangular
struc-ture with the particle size of nanoparticles ranging from 100 to 500 nm. Also, the nanoparticles exhibit polydisperse population and good crystalline features. The interplanar spacing of the nanoparticles were measured as 2.71 Å from
HR-TEM (Fig. 3b) and these correspond to the (200) lattice
planes. As seen in Fig. 3c, the SAED diffraction spots of
the nanoparticles match with the structure of the I-Cu2WS4
and demonstrate that the nanoparticles have a single crys-talline in nature.
Fig. 1 XRD patterns of single phase C2WS4 nanoparticles
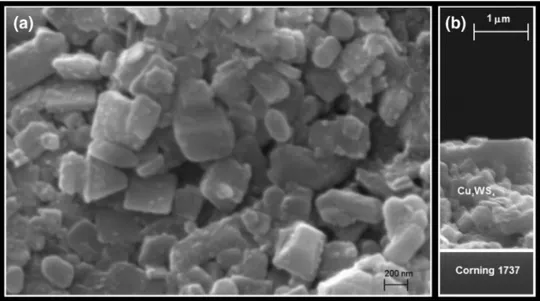
Figure 4 indicates the SEM images of surface and
cross-sectional view of the coated Cu2WS4 thin film. As indicated
in the Fig. 4a, the coated film has, though having hillocks,
acceptable a smooth and uniform surface. The
cross-sec-tional image of the thin film, as shown in the Fig. 4b,which
is the sharp interface between Corning-1737 and Cu2WS4
layer was clearly observed. The film thickness of the nano-particles was measured approximately 1.3 µm.
3.2 Optical and dielectric properties of the Cu2WS4 thin film
Optic constants define the interaction of the emitted light with the material through which the light passes, and
reflection and transmittance determine the optical constants of this material. With the increase of the refractive index, the speed of light in the material decreases. Since the fre-quency is fixed, a reduction in wavelength with a decrease in speed occurs. The extinction coefficient of the wave describes the energy lost in the material and the environ-ment is directly related to the absorption coefficient.
To characterize the basic optical properties such as absorp-tion coefficient (α), refractive index (n), extincabsorp-tion coefficient (k), and real and imaginary parts of dielectric constant (ε) of
the Cu2WS4 thin films, we have grown them onto Corning
1737 glass substrates. The optical reflection and transmission changes with wavelength were measured using by UV-VIS-NIR photospectrometer (Jasco V-670 photospectrometer).
Fig. 3 a TEM, b HR-TEM
and c SAED images of C2WS4
nanoparticles
Fig. 4 a Surface and b
cross-sectional view SEM images of the Cu2WS4 thin film
The spectra were recorded by taking a similar Corning 1737 glass as the reference and hence only the transmission due to
the film was obtained. The optical properties of the Cu2WS4
films were studied at wavelengths from 200 to 2400 nm. The details of the characterization result are discussed below.
The transmittance (T) and reflectance (R) spectrum of the
Cu2WS4 thin film were estimated for the normal incidence
of light between 200–2400 nm at room temperature (300 K). The dependence of the both of Tand R spectra on the
wave-length of the Cu2WS4 thin film is shown in Fig. 5. It is clear
that the optical T in 400–700 nm was estimated in the range
42%. Moreover, the T % 50 for Cu2WS4 is gradually
increas-ing with the increase in wavelength. The upward curvature in reflectance is not combatable with the downward curva-ture in the transmittance at 200–400 nm region which can be ascribed to the light wave scattering due to the nanostructure property of the films. The reflectance of the films falls down rapidly at the transmittance edge, and then increases slightly with increasing wavelength before starting to decrease at wavelength value about 600 nm.
The linear absorption coefficient has been determined by
using both T and R spectra of from the relation [22, 23]
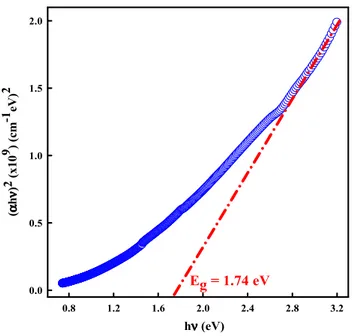
where d is the thickness of film. The direct optical band
gap (Eg) could be calculated from the linear region at the
plot of (𝛼hv)2 versus (hv) (Fig. 6). Optical band gap energy
of Cu2WS4 thin films that were calculated from this linear
region is found as 1.74 eV which is very close that of the
reported literature [11, 12]. The Eg, of thin films is
calcu-lated using the Tauc relation [24]. The relation between
(1) 𝛼= 1 dln ( (1 − R)2 T )
the absorption coefficient, α, and the energy of the incident
light, hv, can be written as follows [25].
where 𝛼0 is a constant and EU is the Urbach energy. To
determine of Urbach tail, we have plotted the variation of
ln𝛼 with hv in Fig. 7. From the slope of linear part of graph,
the Urbach energy,EU, has been calculated as 1.80 eV,
which is quite greater than the value obtained for Cu2WS4
(2) 𝛼(hv) = 𝛼 0exp( hv EU) Wavelenght (nm) 200 400 600 800 1000 1200 1400 1600 T 0.00 0.10 0.20 0.30 0.40 0.50 0.60 R 0.040 0.042 0.044 0.046 0.048 0.050 0.052 0.054 0.056 0.058 T R
Fig. 5 Optical transmittance and reflectance spectra of Cu2WS4 thin film as a function of wavelength
hν (eV) 0.8 1.2 1.6 2.0 2.4 2.8 3.2 x10 (α hν )2 ( 9 ) (c m -1 eV )2 0.0 0.5 1.0 1.5 2.0 Eg = 1.74 eV
Fig. 6 The variation of (𝛼hv)2with photon energy
hν (eV) 1.5 1.6 1.7 1.8 ln α cm -1 ) ( 4.332 4.336 4.340 4.344 4.348 4.352
thin film. This result is most probably due to the great disordered nature of films and also localized states lying deeply between the forbidden band gaps.
The n and the k can be calculated by using the R
accord-ing to followaccord-ing equations [22–26]:
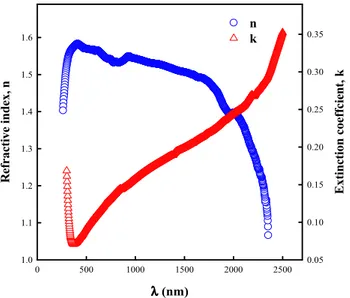
Figure 8 illustrates the dependence of both k and n on
wavelength λ in the UV-VIS-NIR region of the dependence
of the Cu2WS4 thin film. Since R is a dominant factor in the
calculation of n, the behavior of n depending on λ is similar
to that of R (Fig. 5) but the values of both at the same λ are
different. The decrease in n with λ shows the normal dis-persion behavior of the material. The average value of n for
the Cu2WS4 in the most of the visible region of the
spec-tra is nearly 1.55. On the other hand, the k shows a slight increase in the range of wavelength from 400 to 2400 nm.
The k as a function of wavelength for Cu2WS4 thin films is
shown in Fig. 8. This trend is in agreement with the
behav-ior of the absorption coefficient since α is a dominant factor in the calculation of k. The spectral behavior of n is quite similar to that of reflection spectrum as can be expected
from the Eq. 3.
The wavelength of optical properties of the materials dependency is defined as dispersion. The frequency of the light changes as the values of the optical constants of the materials also vary. A general behavior is typical of opti-cal materials’ transparency in the visible, ultraviolet and
k=𝛼𝜆 ⟋4𝜋 (3) n= (1+ R 1− R ) + √ 4R (1 − R)2 − k 2
infrared spectral region and the range of the permeability of the materials in that region without vibrational electronic absorbtion can be determined. The relationship between the
n of the frequency or wavelength “dispersion relation” is
referred to as n and k wavelength depends on. Wavelength spectrophotometric or ellipsometric measurements of the films connected to the optical mirror and optical transmit-tance of the curves after the measurements were taken, someusing optical models of the films thickness, n, k, the range of optical properties such as absorption coefficient and energy band can be found. To be applied optical model vary according to the type and characteristics.
The dispersion of optical parameters can possess a very crucial role in the research of optical materials due to its essential importance at some applications such as optical communication, photovoltaic and several optoelectronic
device designs [27]. For certain purposes, multilayer
opti-cal coatings, deep ultraviolet entire optiopti-cal spectrum from the region up to the infrared region can be designed. The design of optical coatings, the coating on the light the effects of interference due to reflections between successive layers in order to obtain the intended optical response of the layer refractive indices and the method of determination of thickness, depending on the wavelength, optical mirror to be achieved by calculating requires. Graphical representa-tions of the surface and volume energy loss funcrepresenta-tions were also given. As far as we know, any detailed information for
optical constant and dispersion parameters of Cu2WS4
nan-oparticles thin films has not been available in the literature. Therefore, we believe that this work could be valuable for the researchers studying this material.
To represent the n below the optical band gap in terms of energy (or wavelength), the single term Wemple DiDomen-ico oscillator formulae is widely used. Thus, the dispersion
relation for the n can be given by [23, 28];
where Ed is the dispersion energy, which can be interpreted
as a measure of the strength of the interband optical
transi-tion, and E0 is termed as single oscillator energy [29, 30].
If we plot (n2− 1)−1
versus (hv)2, a straight linear region is
obtained, as shown in Fig. 9. The values found for E0 and
Ed are 4.13 and 5.19 eV, respectively. It seems that, the
sin-gle oscillator energy, E0, obeys an empirical relation such
as E02.4 Eg. The static refractive index, n0 can be
calcu-lated by n0=
√
1+ Ed∕E0 as 1.50.
Moreover, the dependent of the moments of the
imagi-nary part of the optical spectrum M−1and M−3 moments can
be determined from the following relations E2
0= M−1∕M−3
and E2
d= M
3
−1∕M−3 [23, 31]. The values of M−1 and M−3
moments are determined as 1.26 and 0.074 eV2
respec-tively by this equation. According to Wemple DiDomenico (4) (n2− 1)−1 = E0 Ed − 1 E0Ed (hv)2 λ(nm) 0 500 1000 1500 2000 2500 Re fractive index, n 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Extinction coeffcie nt , k 0.05 0.10 0.15 0.20 0.25 0.30 0.35 n k
Fig. 8 Refractive index and extinction coefficient variation of
formula [31, 32] namely the oscillator strength ( f ) is given
as f = E0Ed. For Cu2WS4 thin film, f is determined as
21.43 eV2.
In long wavelength region, the dispersion of ncan also be evaluated using the following single term Sellmeier
disper-sion relation [23, 31]
where 𝜆0 is defined as the average interband oscillator
wavelength and n0 is static refractive index. This equation
can be rearranged to give
where S0 is the average oscillator strength and defined as
S0=(n2
0− 1)∕𝜆
2
0 [23, 31]. We have plotted (n
2− 1)−1
versus 𝜆−2 graph in Fig. 10.We have determined n
0,𝜆0
and S0 parameter as 1.49, 300 nm and 1.38 × 10−5 nm−2,
respectively. The value of n0 (1.49) is nearly same to those
obtained above from n0=
√
1+ Ed∕E0.
The following relations have been used to calculate the
values of the 𝜀1 and 𝜀2 of the dielectric constant for Cu2WS4
film as 𝜀1 = n2− k2; 𝜀2= 2nk [23, 26]. We have plotted the
𝜀
1 and 𝜀2 parts of dielectric function of photon energy,
cal-culated from n and k values, as a function of wavelength, in
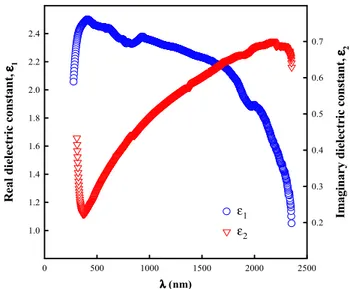
Fig. 11. Figure 11 shows the variation of both 𝜀1 and 𝜀2
val-ues for the Cu2WS4 as a function of photon energy. It can
be seen from the figure that, the spectrum of 𝜀1 is
character-ized by the presence of peak of 2.504 at 412 nm. On the (5) n2− 1 n2 0− 1 = 1 − ( 𝜆 0 𝜆 )2 (6) n2− 1 = S0𝜆 2 0 1− 𝜆2 0∕𝜆2
other hand, the minimum value of 𝜀2 is 0.223 at 370 nm. In
this context, 𝜀1 can be expressed including the free carrier
contribution by following relation [26, 33];
where 𝜀∞ is the high frequency dielectric constant, e is the
elementary charge, c is the speed of light, 𝜀0 is the vacuum
permittivity, N is the free carrier concentration and m∗ is
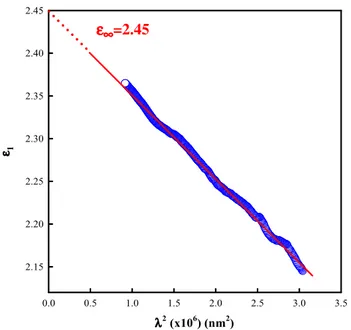
the effective mass. In Fig. 12, the variation of 𝜀1 with 𝜆2 has
been shown. Then, by using intercept at vertical axis of the (7) 𝜀 1= 𝜀∞− ( Ne2 4𝜋2c2𝜀 0m∗ ) 𝜆2 (hυ) (eV)2 2 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 (n 2 -1 ) -1 0.715 0.720 0.725 0.730 0.735 0.740 0.745 0.750
Fig. 9 Variation of plot (n2− 1)−1
with (hv)2, of Cu 2WS4 thin film λ–2 (x10-6) (nm-2) 4.0 4.5 5.0 5.5 6.0 6.5 (n 2 -1 ) -1 0.75 0.76 0.77 0.78 0.79 0.80 Fig. 10 Variation of (n2− 1)−1 with 𝜆−2 of Cu 2WS4 thin film λ nm) 0 500 1000 1500 2000 2500 Re al dielectric constant, ε1 1.0 1.2 1.4 1.6 1.8 2.0 2.2 2.4 Im aginary di el ec tr ic constant , ε2 0.2 0.3 0.4 0.5 0.6 0.7 ε1 ε2 (
Fig. 11 The real (𝜀1) and imaginary (𝜀2) part of dielectric function of
obtained straight line, we have determined 𝜀∞,parameter as
2.45.
In dielectric theory, a dissipation (or loss) fac-tor is defined, to estimate the power loss rate, such as
tan𝛿= 𝜀2∕𝜀1 [23]. In Fig. 13, we have plotted the variation
of dissipation factor with h. It is seen from the figure that the dissipation factor has a small change in magnitude over whole spectral range, varied between 0.9–0.24. At higher
energies, it decreases to 0.9 located nearly at 3.33 eV and increases slightly by increases of energy.
The energy loss is related to the optical properties of the material through the dielectric function. The probabil-ity that the fast electrons will loss energy when traveling the bulk and the surface of the material is defined as the
volume and surface energy loss functions [31].Therefore a
volume (or bulk) of energy loss function can be defined as
[23]
Similarly, low energy electrons can be experienced to an inelastic scattering on the surface of materials. Therefore, inelastic scattering of low-energy electrons can be repre-sented by a surface energy loss function as following,
In Fig. 14, we have indicated the variation of volume
and surface energy loss function with the photon energy. As shown in the figure, two curves have quite similar
spec-tral behavior; as it is clear from the Fig. 14 that, there is
no significant difference between them at lower and higher
photon energies but the VEL increases more than SEL. They
increase slightly from minimum values at higher energy part of the spectrum, nearly at 3.4 eV. In additions, the energy loss by the free charge carriers when traversing the bulk material has approximately the same value as when traveling the surface in particular for relatively lower ener-gies but the energy lost by the bulk volume of such films
(8) VEL= Im { −1 𝜀(𝜔) } = 𝜀 2 𝜀2 1+ 𝜀 2 2 (9) SEL = Im { −1 𝜀(𝜔) + 1 } = 𝜀2 (𝜀1+ 1)2+ 𝜀2 2 x10 λ2 6) (nm2) 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 ε1 2.15 2.20 2.25 2.30 2.35 2.40 2.45 ( ε∞=2.45
Fig. 12 The variation of real part of dielectric coefficient (𝜀1)with 𝜆2
hν (eV) 1.0 1.5 2.0 2.5 3.0 3.5 4.0 tan δ 0.08 0.10 0.12 0.14 0.16 0.18 0.20 0.22 0.24 0.26
Fig. 13 Spectral dependence of the loss factor (tan𝛿) of Cu2WS4 thin
film hν (eV) 0 1 2 3 4 5 Vo lu me energy loss, VEL 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40 0.45 Surface energy loss, SEL 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40 0.45 VEL SEL
Fig. 14 The variation of volume energy loss (VEL) and surface energy loss (SEL) with the photon energy (hv) for Cu2WS4 thin film
is greater than that of the lost by the surface at higher energies.
4 Conclusions
In this study, we have reported that detailed analysis to
determine optical properties of Cu2WS4 as a thin film
which synthesized Cu2WS4 nanoparticles were
synthe-sized via hot injection method. Cu2WS4 thin films were
produced by a spin coating method on Corning 1737
sub-strates in atmosphere medium. Formation of Cu2WS4
achieved layered Cu2WS4 structures with uniform content
at about 1.3 µm. With spin coating settings homogenous
Cu2WS4 thin films can be achieved. The optical band gap
of films is 1.74 eVwhich are comparable to experimental
values reported in the earlier study for Cu2WS4. X-ray
dif-fraction technique energy dispersive X-ray (EDS-EDX), scanning electron microscopy and atomic force
micros-copy techniques were used to characterize Cu2WS4 thin
films. Cu2WS4 layer uniformly distributed to whole films
structures indicating that films stoichiometry is well dis-tributed. The using energy dispersive X-ray analysis (EDS) measurements investigated the chemical composition of
source and deposited Cu2WS4 thin films. On the principle
of EDX analysis, it was found that the grown films were stoichiometric which was also substantiated by structural analysis.As mentioned above,our calculated optical
param-eters of the nanoparticles Cu2WS4 thin film are unique.The
Urbach energy of the Cu2WS4 thin film has been calculated
as 2.90 eV. From the wavelength dependence of k and n
of the Cu2WS4 thin film, a peak of the n of 1.58 appears
at 410 nm. The minimum value of the k is 0.07 at 374 nm and decreases slowly in the ranging of 374–1500 nm.The
optical dispersion parameters ofthe Cu2WS4can be by the
single oscillatormodel of Wemple DiDomenico and single term Sellmeier dispersion relations where the single
oscil-lator energy(E0), the dispersion energy (Ed), static
refrac-tive index (n0), the average interband oscillator wavelength
(𝜆0), and the average oscillator strength (S0) are 4.13 eV,
5.19 eV, 1.50, 300 nm and 1.38 × 10−5 nm−2 respectively.
Furthermore, according to dielectric characterizations, the
high frequency dielectric constant, 𝜀∞ is found as 2.45. The
variation of the dielectric constants with the photon ener-gies shows that there are some interactions between
pho-tons and electrons in the Cu2WS4 thin film that is studied
over an energy range of 0.6–2 eV. The consequences are dedicated to the morphology and structure of nanoparticle
films of hopeful substance as Cu2WS4 may be useful for
experts and researchers. These kinds of grown films have also large applications of semiconductor in different opto-electronic tools such as photovoltaic and imaging tools.
Acknowledgements We want to thank to Karamanoglu Mehmetbey
University, Scientific Research Foundation for (32-M-16) financial support of this work. The authors would like to thank TUBITAK (The Scientific and Technological Research Council of Turkey) (215M309) for supporting this work.
References
1. K.M. Ryan, S. Singh, P. Liu, A. Singh, CrystEngComm 16, 9446–9454 (2014)
2. Y. Xie, L. Carbone, C. Nobile, V. Grillo, S. D’Agostino, F.D. Sala, C. Giannini, D. Altamura, C. Oelsner, C. Kryschi, P.D. Cozzoli, ACS Nano 7, 7352–7369 (2013)
3. H. Tong, N. Umezawa, J. Ye, T. Ohnoc, Energy Environ. Sci. 4, 1684–1689 (2011)
4. J. Lia, N. Wu, Catal. Sci. Technol. 5, 1360–1384 (2015) 5. J. Luxa, O. Jankovský, D. Sedmidubský, R. Medlín, M. Maryško,
M. Pumerad, Z. Sofer, Nanoscale 8, 1960–1967 (2016)
6. P.D. Tran, M. Nguyen, S.S. Pramana, A. Bhattacharjee, S.Y. Chiam, J. Fize, M.J. Field, V. Artero, L.H. Wong, J. Loo, J. Bar-ber, Energy Environ. Sci. 5, 8912–8916 (2012)
7. E.A. Pruss, B.S. Snyder, A.M. Stacy, Angew. Chem. Int. Ed. Engl. 32, 256–257 (1993)
8. C.J. Crossland, J.S.O. Evans, Chem. Commun 18, 2292–2293 (2003)
9. C.J. Crossland, P.J. Hickey, J.S. Evans, J. Mater. Chem. 15, 3452–3458 (2005)
10. T.F. Jaramillo, K.P. Jorgensen, J. Bonde, J.H. Nielsen, S. Horch, I. Chorkendorff, Science 317, 100–102 (2007)
11. D. Jing, M. Liu, Q. Chen, L. Guo Int, J. Hydrogen Energy 35, 8521–8527 (2010)
12. N. Li, M. Liu, Z. Zhou, J. Zhou, Y. Sun, L. Guo, Nanoscale 6, 9695–9702 (2014)
13. L.Y. Gan, U. Schwingenschlögl, Phys. Rev. B 89, 125423 (2014) 14. J.P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 77, 3865
(1996)
15. S. Grimme, J. Comput. Chem. 27, 1787–1799 (2006)
16. J. Heyd, G.E. Scuseria, M. Ernzerhof, J. Chem. Phys. 124, 219906 (2006)
17. J. Heyd, G.E. Scuseria, M. Ernzerhof, J. Chem. Phys. 118, 8207 (2003)
18. S.H. Chaki, M.P. Deshpande, J.P. Tailor, Thin Solid Films 550, 291–297 (2014)
19. S.H. Chaki, J.P. Tailor, M.P. Deshpande, Adv. Sci. Lett. 20, 959– 965 (2014)
20. A.F. Al-Hossainy, A. Ibrahim, Mater. Sci. Semicond. Process.
38, 13–23 (2015)
21. F. Ozel, E. Aslan, A. Sarilmaz, İ.H. Patir, ACS Appl. Mater. Interfaces 8, 25881–25887 (2016)
22. N. Tuğluoğlu, B. Barış, H.G. Özdemir, S. Karadeniz, Ö.F. Yük-sel, J. Alloys Compd. 582, 696–702 (2014)
23. B. Barış, H.G. Özdemir, N. Tuğluoğlu, S. Karadeniz, Ö.F. Yük-sel, Z. Kişnişci, J. Mater. Sci. 25, 3586–3593 (2014)
24. J. Tauc, A. Menth, J. Non Cryst. Solids 8, 569–585 (1972) 25. F. Urbach, Phys. Rev 92, 1324–1324 (1953)
26. M.M. El-Nahass, A.M. Farid, A. Atta, J. Alloy Compd. 507, 112–117 (2010)
27. A.F. Al-Hossainy, A. Ibrahim, Opt. Mater. 46, 131–140 (2015) 28. S.H. WempleJr, M. DiDomenico, Phys. Rev. B 3, 1338–1351
(1971)
29. S.H. WempleJr, M. DiDomenico, Phys. Rev. B 1, 193–202 (1970)
31. A.A.M. Farag, M. Fadel, Opt. Laser Technol. 45, 356–363 (2013)
32. M.M. El-Nahass, H.S. Soliman, A.A. Hendi, S.H. El-Gamdy, Aust. J. Basic Appl. Sci. 5, 145–156 (2011)
33. M. Yıldırım, F. Özel, N. Tuğluoğlu, Ö.F. Yüksel, M. Kuş, J. Alloy Compd. 666, 144–152 (2016)